Method for measuring glycosylated hemoglobin, and device for measuring glycosylated hemoglobin
A technology of glycosylated hemoglobin and hemoglobin, applied in the field of glycosylated hemoglobin, which can solve problems such as difficult to reflect symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
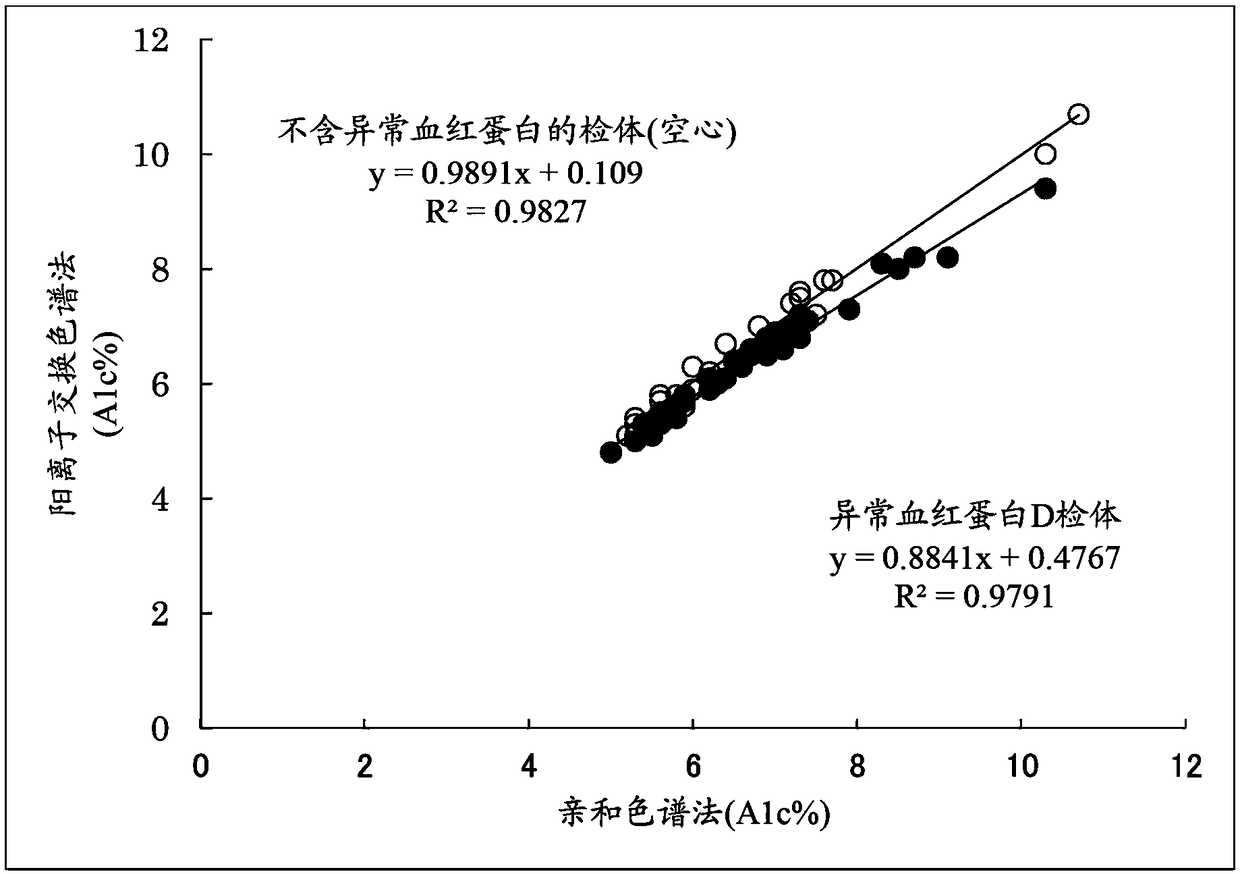
[0111] For blood samples containing abnormal hemoglobin D, the following method of the present invention was implemented: in the chromatogram obtained by cation exchange chromatography ( Figure 5 ), calculate the total peak area (Total area) of the chromatogram and the peak areas of A1a, A1b, HbF, LA1c, sA1c, A', H-V0, and obtain the X1c area according to the formula (3), according to the formula ( 2) Determine the area of A0, and calculate A1c% according to formula (1). It should be noted that the peak detection range of H-V0 is 1.00±0.07 minutes.
[0112] (1) Peak area of A'
[0113] Calculate the peak area of A' according to the chromatogram to be 921.2.
[0114] (2) Peak area of α
[0115] According to the chromatogram, the peak area of α was calculated to be 8.0+6.7+38.1+63.9=116.7.
[0116] (3) Peak area of sA1c
[0117] According to the chromatogram, the peak area of sA1c was calculated to be 63.9.
[0118] (4) Peak area of X0
[0119] Calculate t...
Embodiment 2
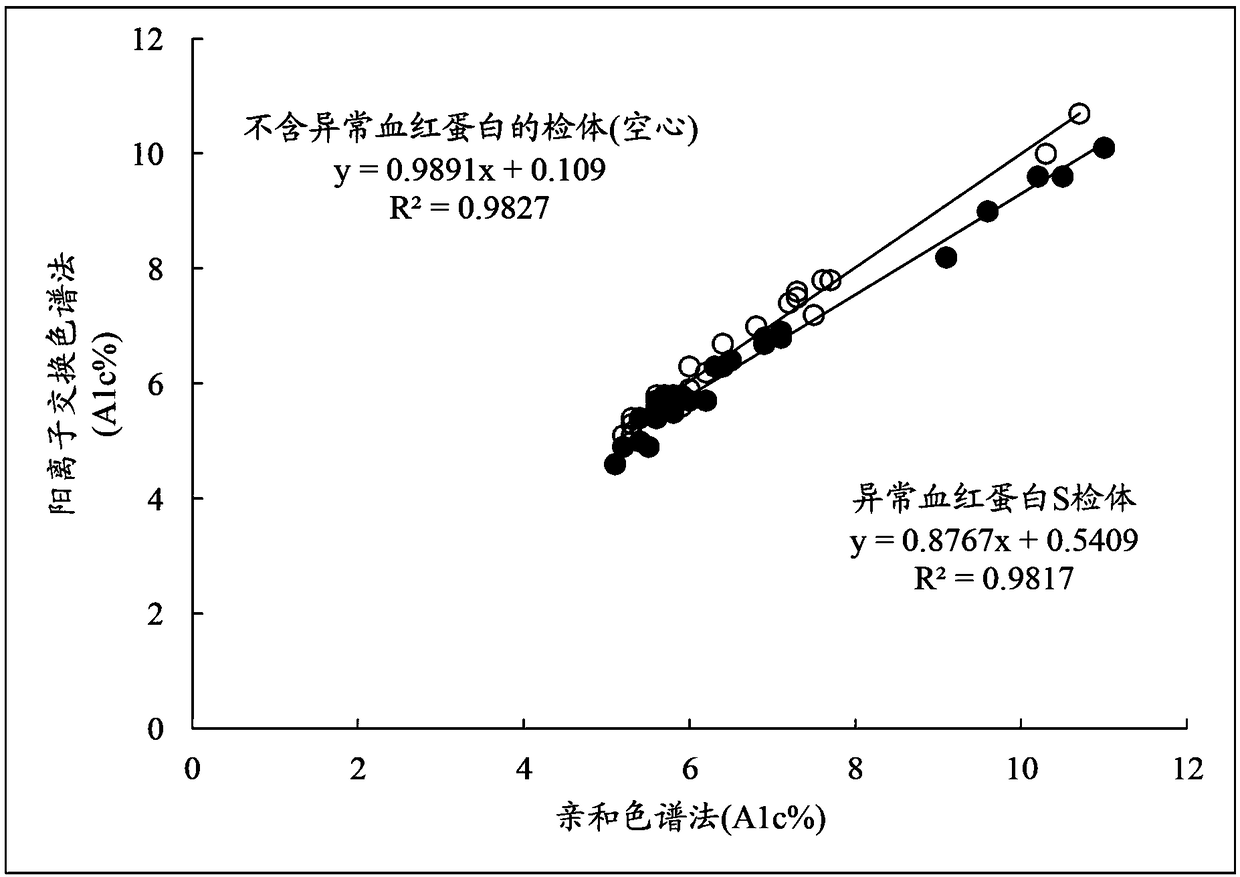
[0131] For blood samples containing abnormal hemoglobin S, the following method of the present invention was implemented: in the chromatogram obtained by cation exchange chromatography ( Image 6 ), calculate the total peak area (Total area) of the chromatogram and each peak area of A1a, A1b, HbF, LA1c, sA1c, A', H-V1, and obtain the X1c area according to the formula (3), according to the formula ( 2) Determine the area of A0, and calculate A1c% according to formula (1). It should be noted that the peak detection range of H-V1 is 1.16±0.09 minutes.
[0132] (1) Peak area of A'
[0133] Calculate the peak area of A' according to the chromatogram to be 899.0.
[0134] (2) Peak area of α
[0135] According to the chromatogram, the peak area of α was calculated to be 11.8+7.8+31.4+78.6=129.6.
[0136] (3) Peak area of sA1c
[0137] According to the chromatogram, the peak area of sA1c was calculated to be 78.6.
[0138] (4) Peak area of X0
[0139] Calculat...
Embodiment 3
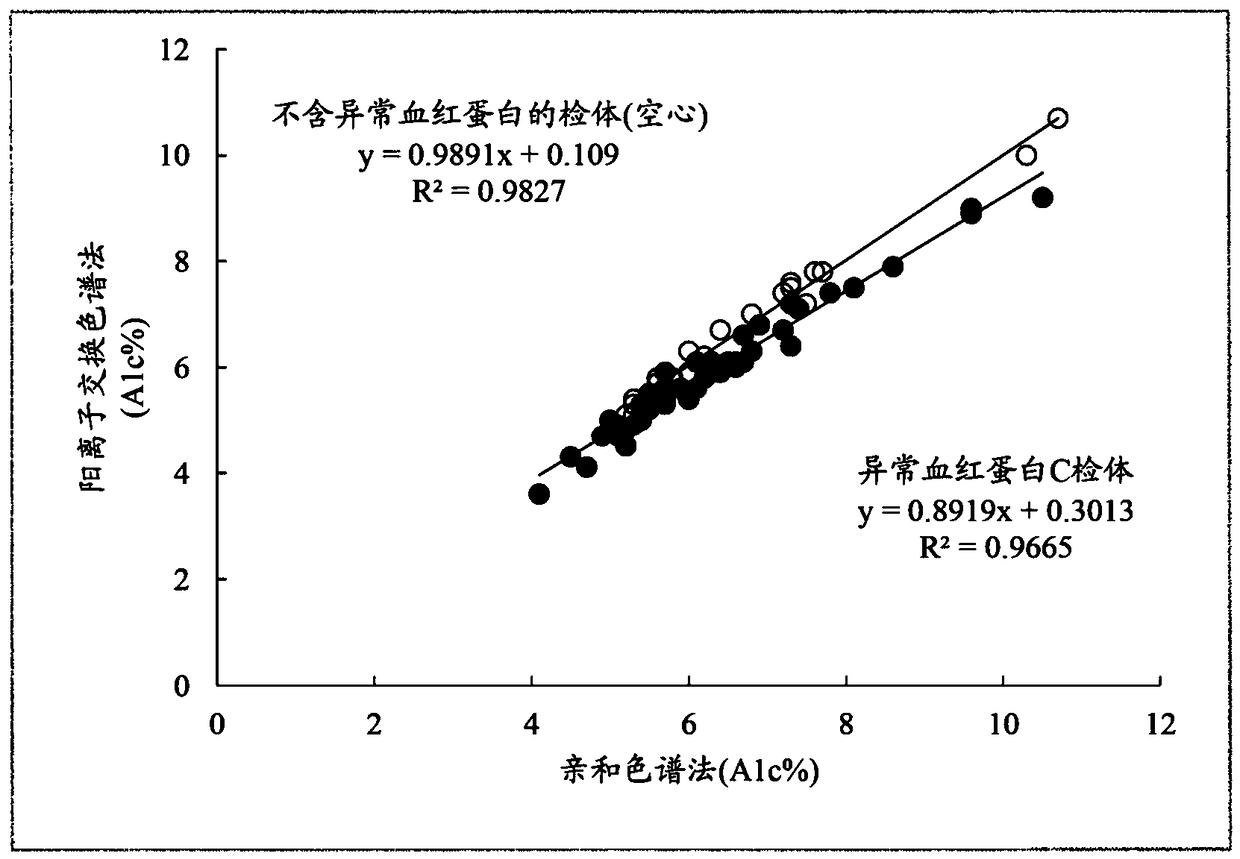
[0151] For blood samples containing abnormal hemoglobin C, the following method of the present invention was implemented: in the chromatogram obtained by cation exchange chromatography ( Figure 7 ), calculate the peak areas of A1a, A1b, LA1c, sA1c, and A0, and calculate A1c% according to formula (4). It should be noted that the peak detection range of H-V2 is 1.34±0.09 minutes.
[0152] (1) Peak area of A0
[0153] According to the chromatogram, the peak area of A0 was calculated to be 1140.3.
[0154] (2) Peak area of α
[0155] According to the chromatogram, the peak area of α was calculated to be 10.8+11.8+34.8+89.9=147.3.
[0156] (3) Peak area of sA1c
[0157] According to the chromatogram, the peak area of sA1c was calculated to be 89.9.
[0158] (8) Calculation of A1c%
[0159] Substituting the numerical value obtained above into formula (4), A1c%=6.7% was calculated.
[0160] (9) Converted to NGSP value (conversion factors 1.1151, 0.6558)
[0161] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com