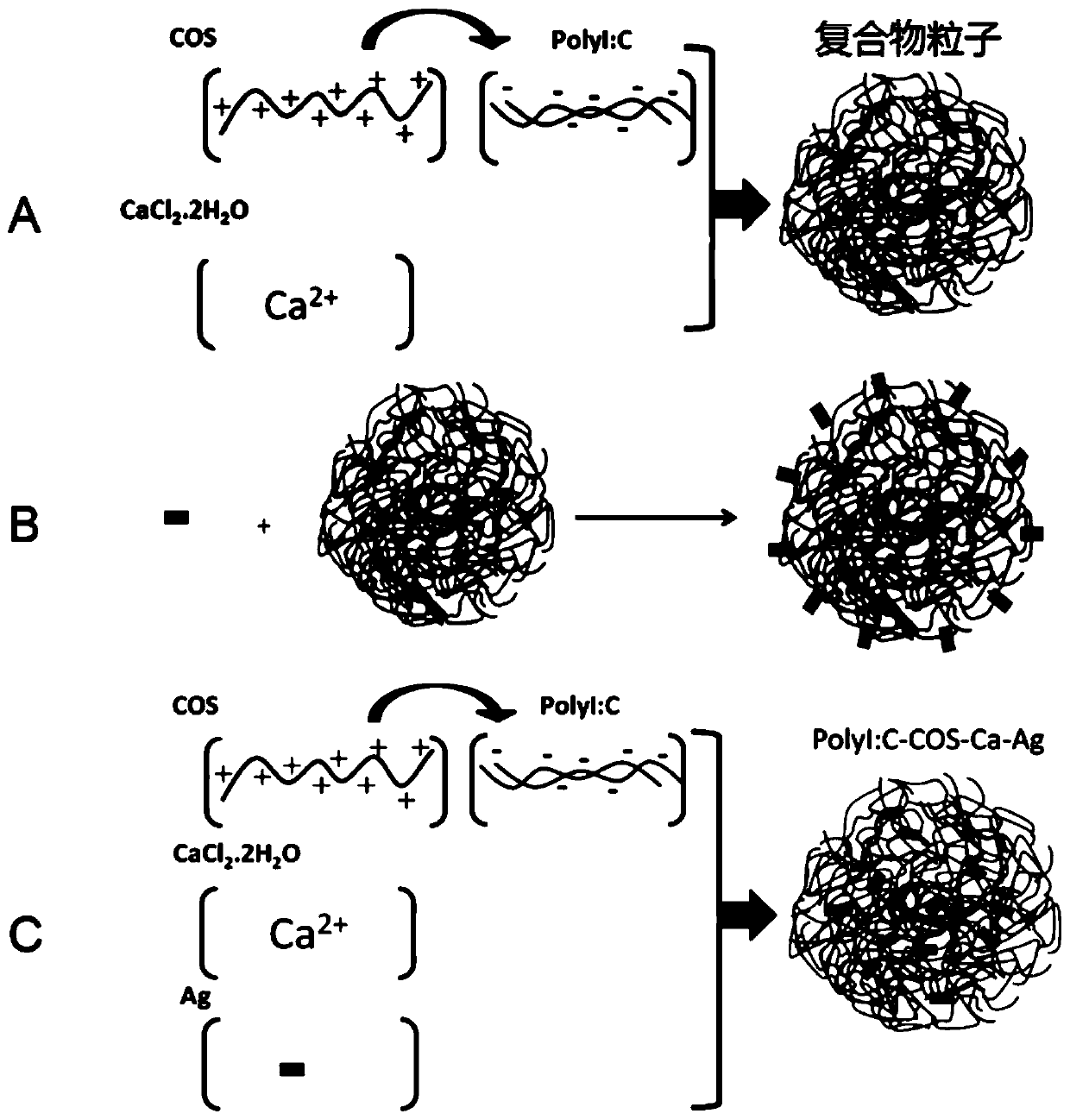
Complex for Enhanced Immune Response
A technology that enhances immunity and complexes, applied in the field of biomedicine, can solve problems such as insufficient safety and effectiveness data, large side effects, and poor effects, and achieve the effects of convenient pharmaceutical preparation, easy absorption, and enhanced immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] According to one aspect of the present invention, the present invention also relates to a method for preparing a compound for enhancing immune response, comprising:
[0160] contacting polyinosinocytes, at least one cationic stabilizer, and a soluble calcium salt in a liquid reaction system;
[0161] The cationic stabilizer is a water-soluble non-antibiotic amino compound with a molecular weight of ≤5kDa, or the water-soluble non-antibiotic amino compound mixed with polyethylene glycol monomethyl ether, polyethylene glycol, polyethyleneimine, folic acid, galactose One or more of the formed grafts.
[0162] In some embodiments, the polyinosinic acid is prepared by base pairing reaction of polycytidylic acid and polyinosinic acid.
[0163] In some embodiments, the molecular weight of the polycytidylic acid and polyinosinic acid is greater than 23,000 Daltons.
[0164] In some embodiments, the polycytidylic acid has a molecular weight ranging from 66,000 Daltons to 660,0...
Embodiment 1
[0191] The preparation of embodiment 1 pamica
[0192] 1. Preparation of Pamika Complex
[0193] 1. Prepare PBS solution (pH7.2):
[0194] 1.1 Prepare sodium chloride solution (0.85%, 1500ml): weigh 12.75g of sodium chloride, put it into a 2000ml measuring cylinder, and dilute to 1500ml with water;
[0195] 1.2 Prepare disodium hydrogen phosphate solution (0.006mol / L, 500ml): weigh disodium hydrogen phosphate 0.006×0.5×141.96=0.4259g, put it into a 500ml volumetric flask, and dilute to 500ml with 0.85% normal saline;
[0196] 1.3 Prepare sodium dihydrogen phosphate solution (0.006mol / L, 500ml): weigh sodium dihydrogen phosphate 0.006×0.5×137.99=0.4140g, put it into a 500ml volumetric flask, and dilute to 500ml with 0.85% normal saline;
[0197] 1.4 Prepare a PBS solution with a pH value of 7.2: take 273.6ml of "1.2 solution" + 126.4ml of "1.3 solution".
[0198] 2. Prepare PIC solution (2.0mg / ml, 100ml):
[0199] 2.1 Weigh PI 2.0mg / ml*100ml*【1.04 / (1.04+1)】 / 91.5% / (1-2.7%)=1...
Embodiment 2
[0280] Embodiment 2 Pamika combined with PEI increases the solubility test
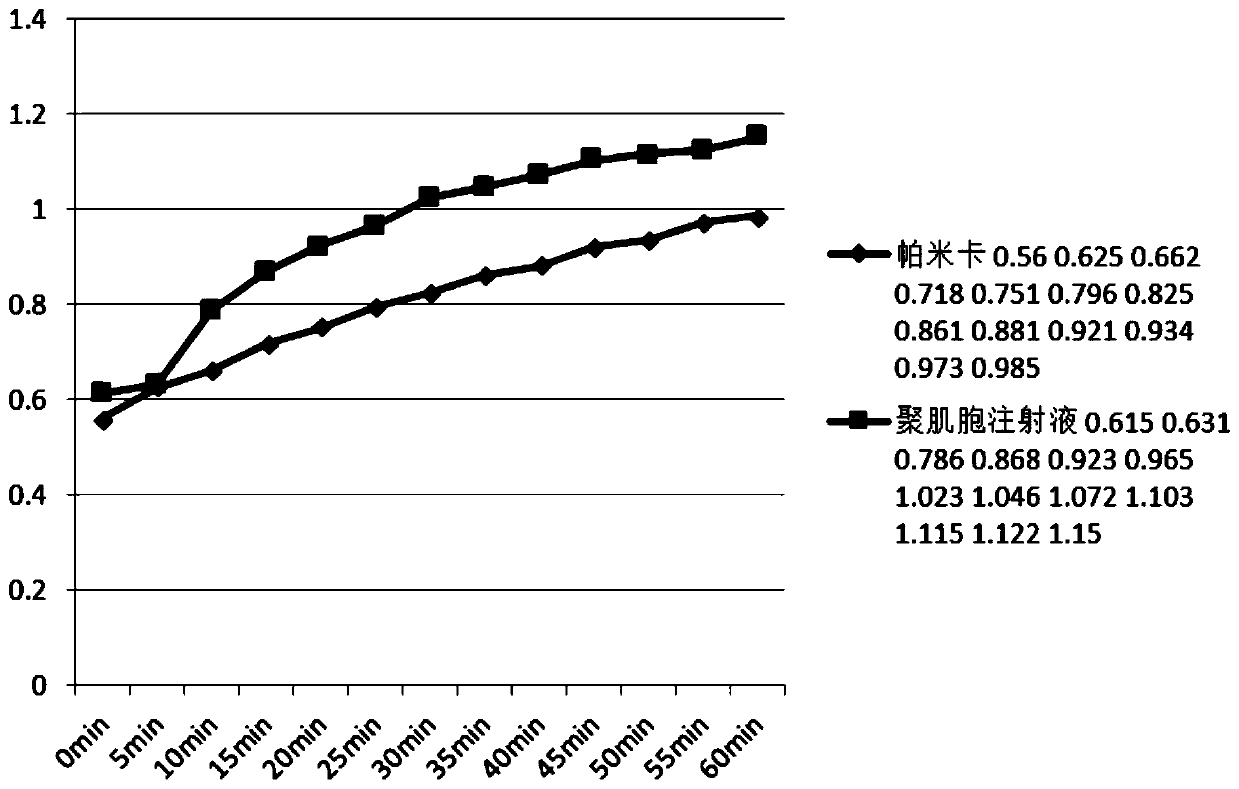
[0281] Refer to the preparation method of "Pamica combined with PEG" (i.e. Example 1) for preparation. Precipitation occurs after increasing the amount of COS in Pamica, which affects the uniformity of its administration. After adding PEI, precipitation can be avoided and COS can be increased. The dosage can further enhance its immune effect.
[0282]
[0283] The test clearly showed that after pamicha combined with PEI, the solubility of COS in PIC increased from 1.6mg / ml to 6.4mg / ml, at least 4 times.
[0284] CN105396130A patent application literature discloses a "calcium dermamine adjuvant and a vaccine containing calcium dermamine adjuvant", and discloses that the non-antibiotic amino compound can be selected as chitosan.
[0285] In this comparative example, water-soluble chitosan (chitosan hydrochloride, referred to as CS) was used to replace the chitosan oligosaccharide in Example 1, so as t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com