Antibodies, uses thereof and conjugates thereof
一种缀合物、抗体的技术,应用在抗体、体内试验用的配制品、抗动物/人类的免疫球蛋白等方向,能够解决反应短暂、没有已显示出提高存活率疗法等问题,达到降低聚集的趋势、良好选择性、促进免疫反应的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0289] Example 1: Production of Antibodies
[0290] Five heavy chain and four light chain sequences (designated VH1 to VH5, and VK1 to VK4, respectively) were selected. Their amino acid sequences are respectively SEQ ID No 10 to 14 and 15 to 18, and their nucleic acid sequences are respectively SEQ ID No 1 to 5 and 6 to 9. The following comparator prior art antibodies were also selected: the heavy and light chain V region sequences of mJ591 (respectively amino acid sequences SEQID No 23 and 24, respectively nucleic acid Seq ID 19 and 20), and deimmunized J591 (respectively Amino acid sequence SEQ ID No 25 and 26, respectively nucleic acid Seq ID 21 and 22).
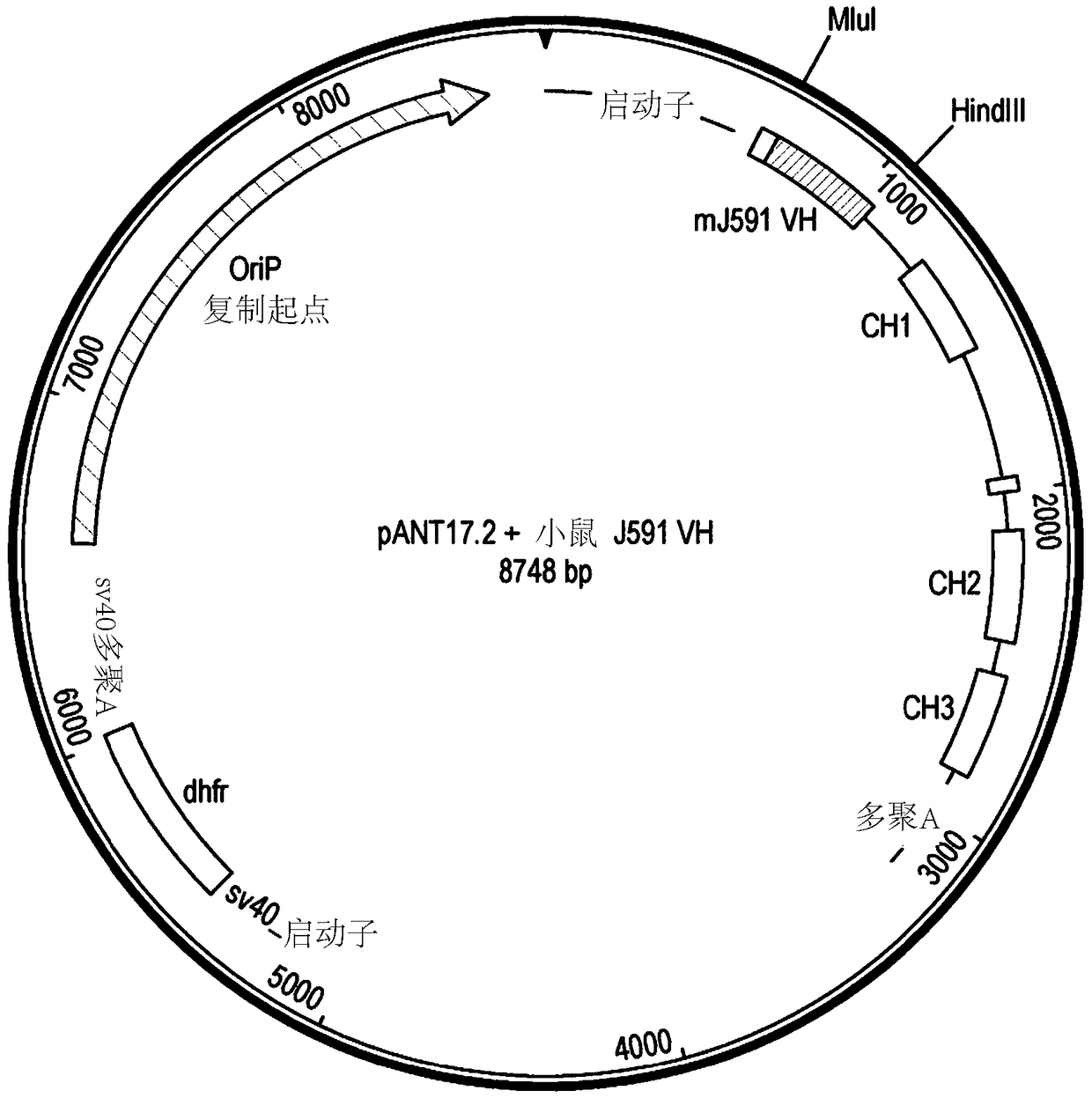
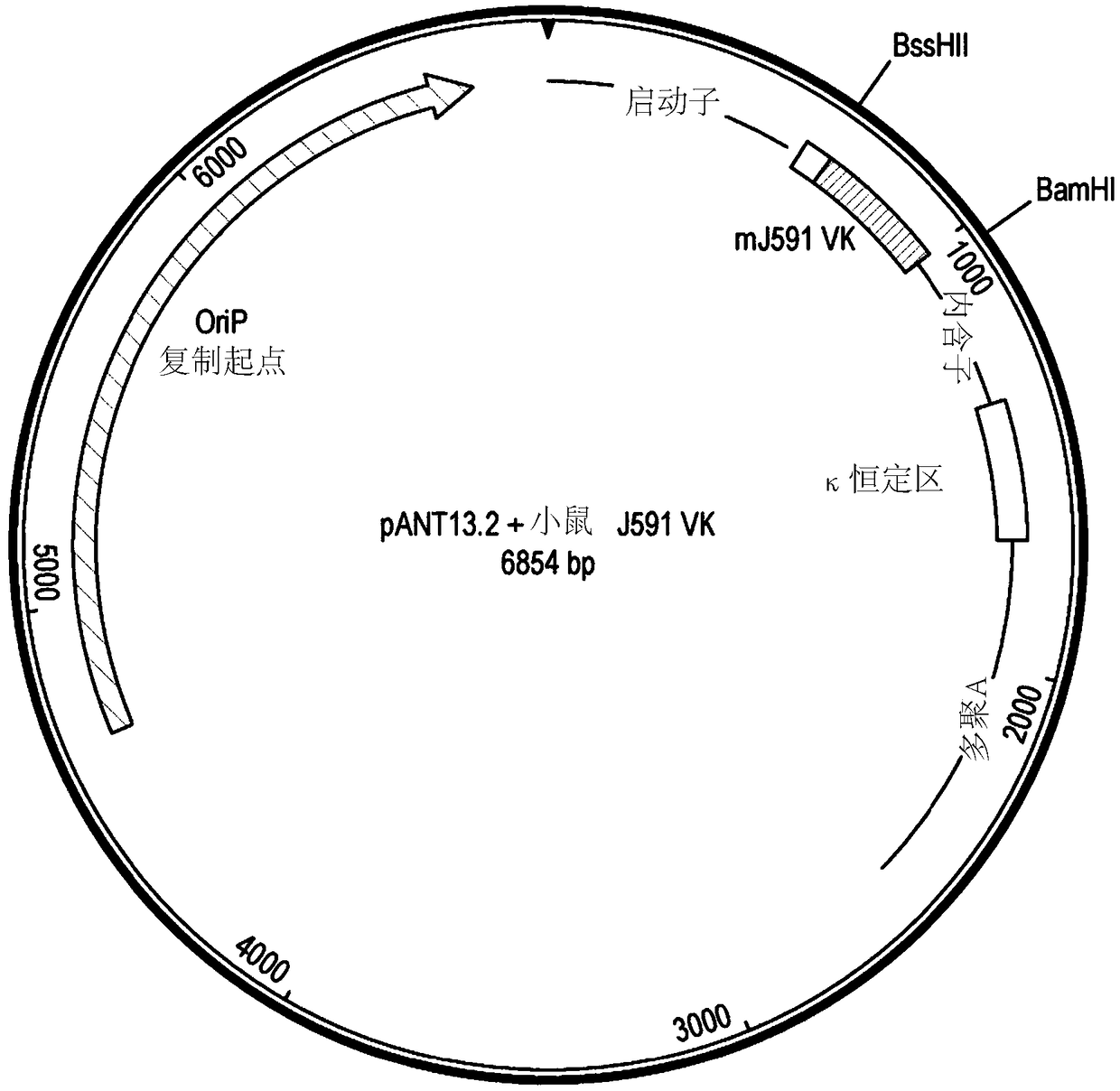
[0291] DNA encoding the variant V region was synthesized and subcloned into the pANT antibody expression vector ( Figure 1a ), wherein the heavy and light chain V regions were cloned into pANT17.2 and pANT13.2, respectively. The heavy chain V region gene was cloned into pANT17.2 via MluI and HindIII sites in frame wit...
Embodiment 2
[0296] Example 2: Generation of NSO cell lines expressing PSMA
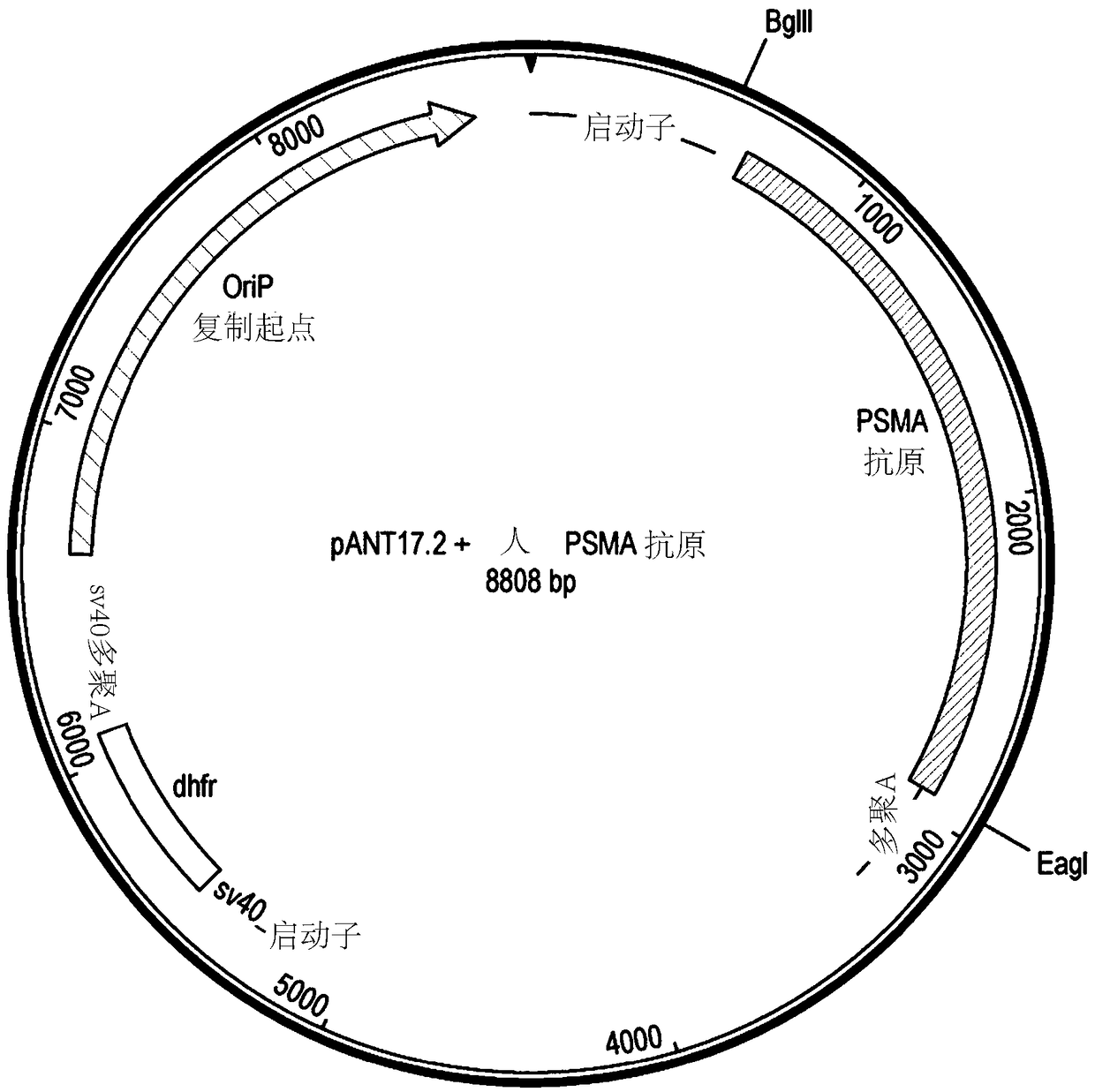
[0297] The full-length human PSMA antigen (FOLH1_HUMAN ECACC Accession No. Q04609) was codon optimized (DNA SEQID No.31, amino acid Seq ID No.32), synthesized and expressed via BglII and EagI sites (IgG1 heavy chain removed) cassette) subcloned into the expression vector pANT17.2 antibody ( Figure 1b ). Transcription of the PSMA gene is under the control of the CMV I / E promoter (US5168062 and US5385839, University of Iowa), and the pANT17.2 plasmid contains the SV40 promoter and polyA sequence for selection in eukaryotic cells. Mutation of the dhfr minigene (Simonsen & Levinson 1983, PNAS 80:2495-2499). pANT17.2 also contains β-lactamase (Ap R ) gene and the pMB1 origin of replication for propagation in prokaryotic cells. All plasmids were propagated in E. coli XL1-blue (Stratagene Cat. No. 200130). The PSMA expression constructs were then stably co-transfected into NSO cells by electroporation, and clones ...
Embodiment 3
[0300] Example 3: Analysis of Humanized Antibodies
[0301] Binding of NSO-derived J591 variants to PSMA antigen was assessed in a competitive FACS ELISA with parental chimeric J591 and deimmunized J591 reference antibodies. Deimmunized J591 antibody was fluorescently labeled using the AlexaFluor 488 Antibody Labeling Kit (MolecularProbes, Paisley, UK). PSMA-expressing NS0 cells (clone 6 / 2F4, 3 × 10 per staining) were harvested 5 cells), washed once with Dulbecco's PBS (PAA Laboratories, Yeovil, UK), resuspended in blocking buffer (PBS containing 1% BSA / 0.05% sodium azide, 2.5% goat serum) and incubated at room temperature Incubate for 30 minutes. Various concentrations of the test humanized antibody were premixed with a constant concentration of Alexa-fluor 488-labeled deimmunized J591 antibody (0.5 μg / mL final concentration). Blocked cells were then resuspended in 150 μL / well of pre-diluted antibody mix and incubated on ice for 1 h. After incubation, the cells were washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com