Active monitoring method and system for adverse drug reactions
A technology of adverse reactions and medicines, applied in medicine or prescriptions, unstructured text data retrieval, text database query, etc., can solve problems such as inability to monitor patients, and achieve the effect of improving work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] In order to have a clearer understanding of the technical features, purposes and effects of the present invention, the specific implementation manners of the present invention will now be described in detail with reference to the accompanying drawings.
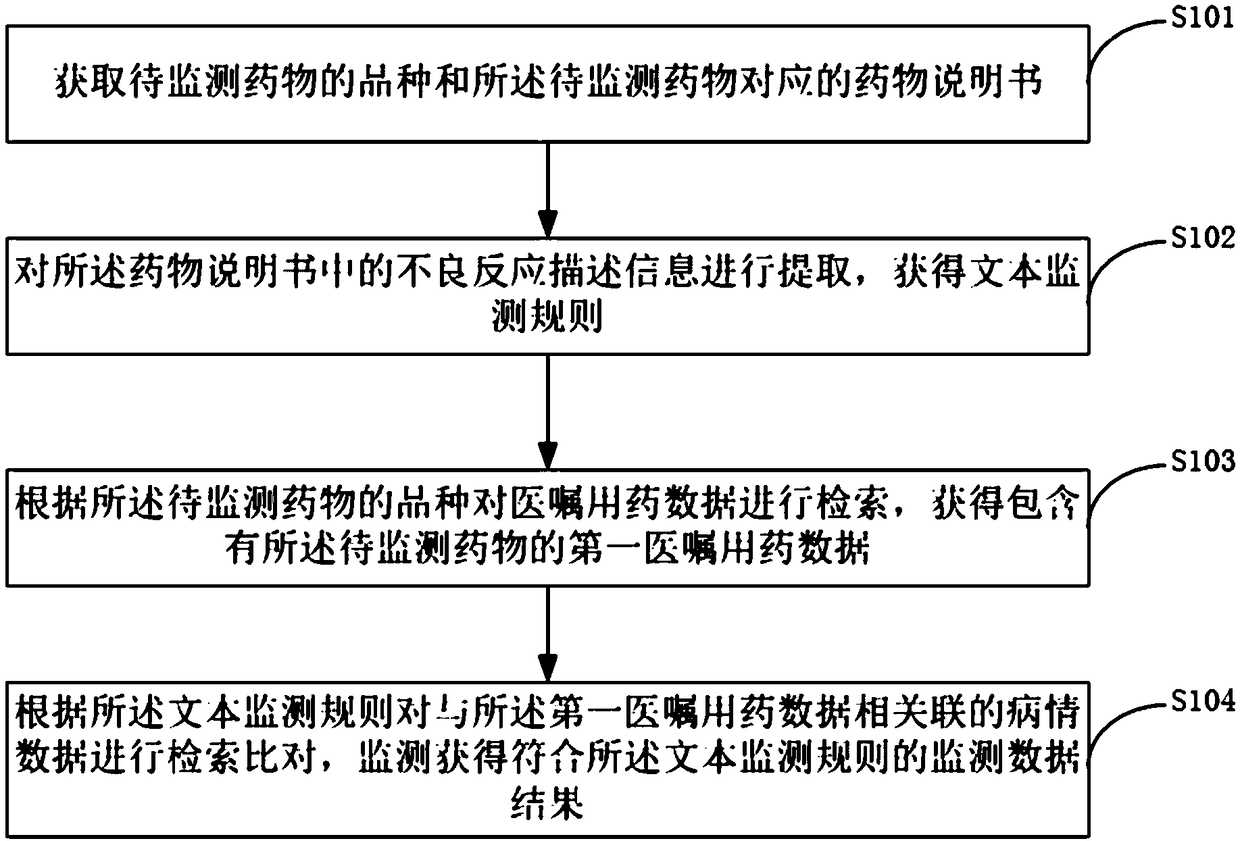
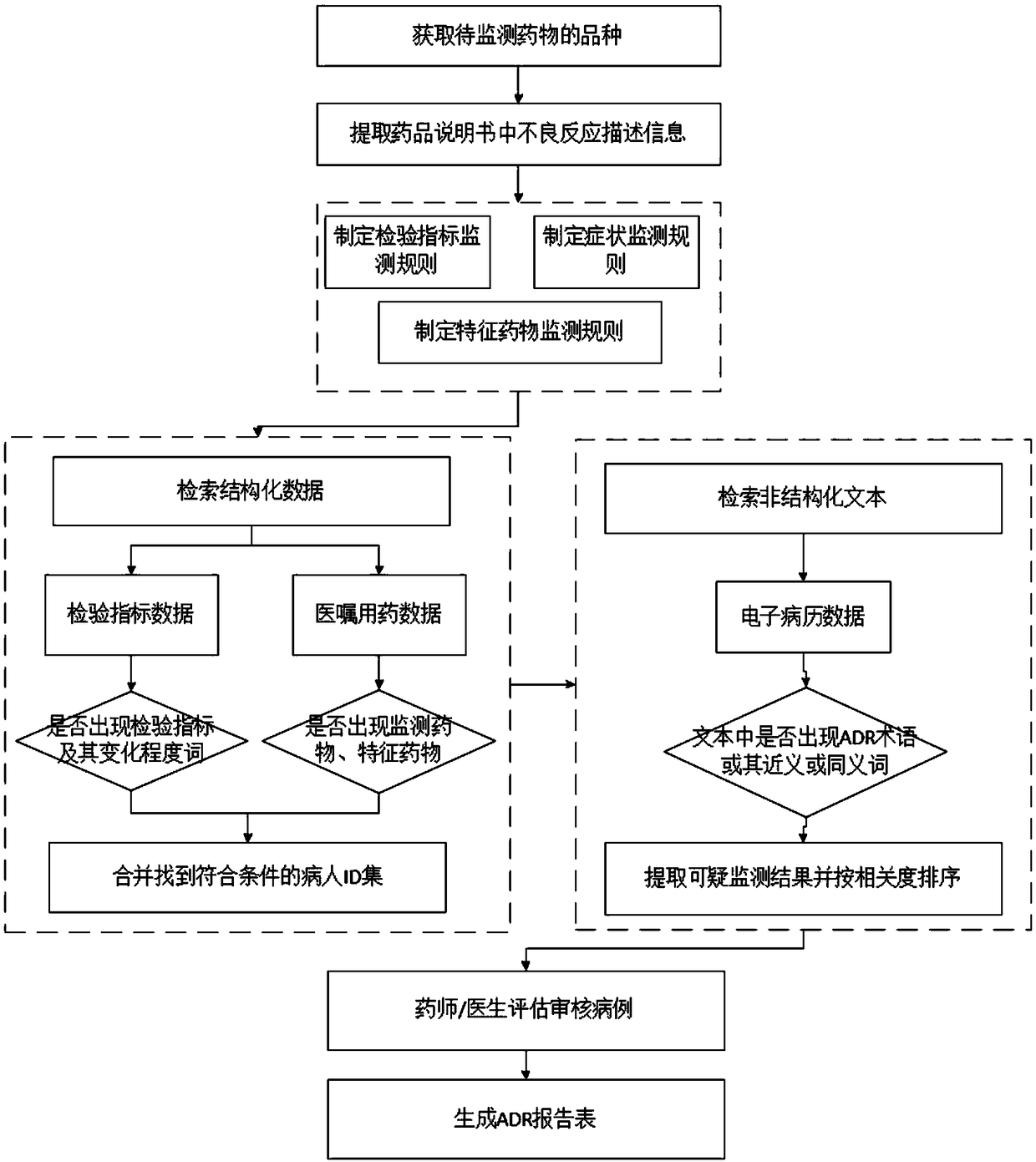
[0050] Such as figure 1 as shown, figure 1 It is a schematic flowchart of an active monitoring method for adverse drug reactions provided by an embodiment of the present invention. The active monitoring method for adverse drug reactions provided by an embodiment of the present invention includes:
[0051] S101. Obtain the type of drug to be monitored and the drug instruction sheet corresponding to the drug to be monitored;
[0052]It is understandable that some drugs that are prone to adverse reactions or have serious consequences after use are usually labeled as high-risk drugs by the hospital; The unknown effects of drugs can be controlled, and some drugs are usually included in the drug safety monitoring project to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com