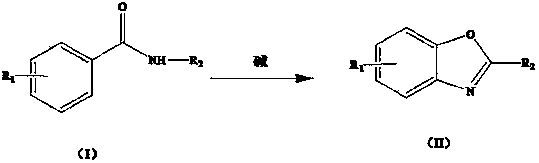
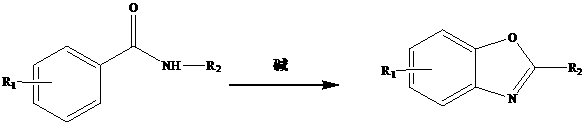Method for synthesizing benzoxazole through microwave radiation of benzamide compound in water phase
A technology for benzamide and benzoxazole, which is applied in the field of synthesizing benzoxazole from benzamide compounds, can solve the problems of energy and environmental burden, poor biodegradability, long reaction time and the like, and achieves environmental friendliness and easy operation. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Benzoxazole: add 2 ml of water, 1 mmol of benzamide and 0.2 mmol of cesium carbonate in a microwave reactor, react for 10 min in a microwave power of 120 W, extract with ethyl acetate, concentrate under reduced pressure, and the product Silica gel column separation gave a white solid in 99% yield.
Embodiment 2
[0020] Embodiment 2: 2-methylbenzoxazole: the preparation method is the same as that of embodiment 1, and what is added is N - methylbenzamide as a white solid in 93% yield.
Embodiment 3
[0021] Embodiment 3: 2-ethyl benzoxazole: preparation method is the same as embodiment 1, what is added is N - Ethylbenzamide as a white solid in 92% yield.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap