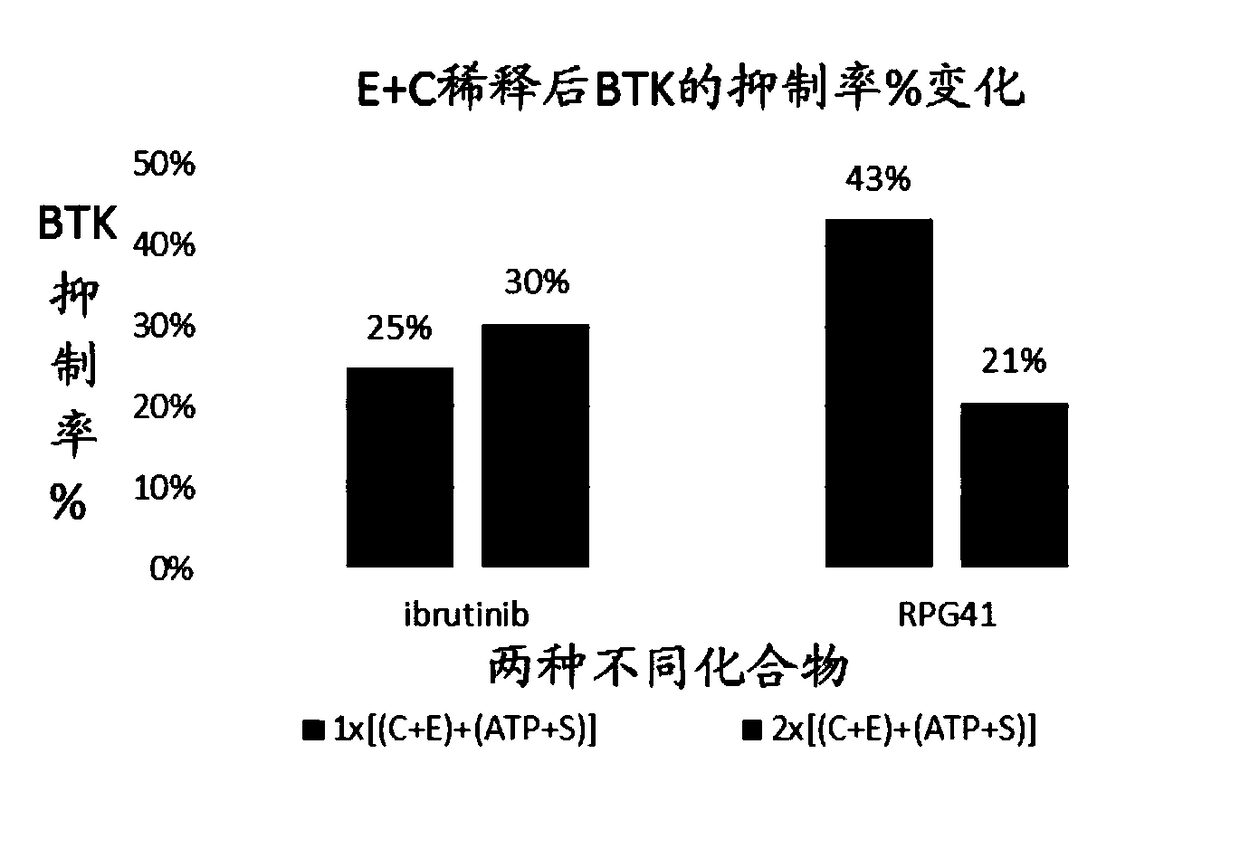
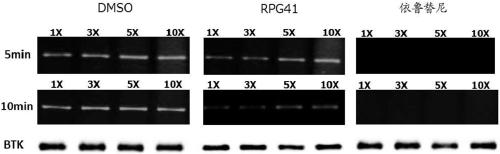
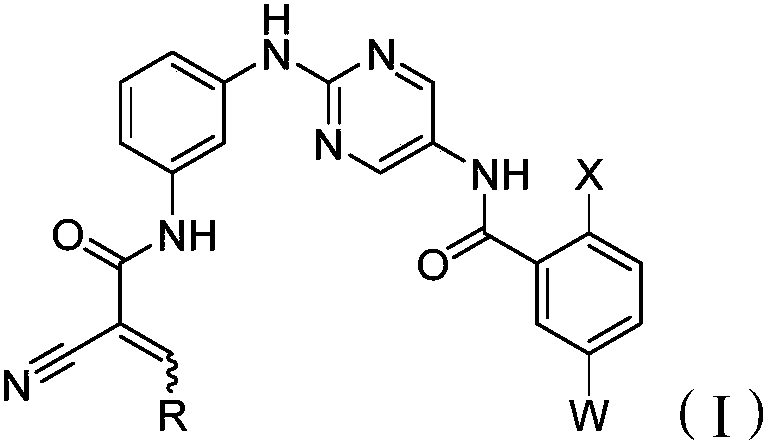
Bruton's tyrosine kinase inhibitor
A technology of amino and compounds, applied in the field of compounds that inhibit the activity of Bruton's tyrosine kinase in a covalent and reversible manner, can solve the problem that reversible covalent inhibitors do not yet exist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0100] The following specific non-limiting examples will be construed as merely illustrative and do not limit the present invention in any way. Although no further detailed description is required, it is believed that those skilled in the art can fully utilize the present invention based on the description herein.
[0101] Compound synthesis
[0102] In the following synthesis schemes, the following abbreviations are used:
[0103] Boc: tert-butoxycarbonyl;
[0104] Et 3 N / TEA: Triethylamine;
[0105] Dioxane: 1,4-dioxane;
[0106] RT: room temperature;
[0107] CH 3 CN: Acetonitrile;
[0108] K 2 CO 3 : Potassium carbonate;
[0109] SOCl 2 :Thionyl chloride;
[0110] DCM: dichloromethane;
[0111] DIEA: N,N-diisopropylethylamine;
[0112] HATU: 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate;
[0113] DMF: Dimethylformamide;
[0114] TFA: Trifluoroacetic acid
[0115] step 1:
[0116]
[0117] Combine m-phenylenediamine 1 (0.500g, 4.62mmol) and (Boc) 2 O (0.92mL, 4.0mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com