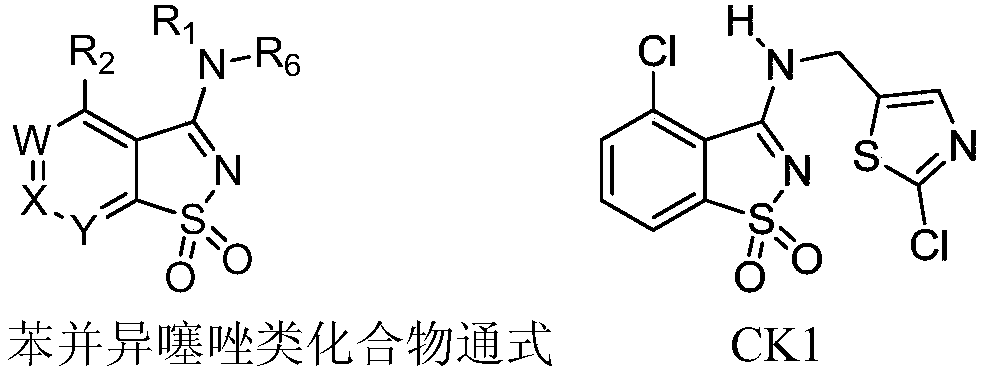
Substituted benzisothiazole compound and preparation method and application thereof
A technology for benzisothiazoles and compounds, which is applied in the field of substituted benzisothiazoles and their preparations, and can solve the problems that structural benzisothiazoles have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Example 1: 3-(((2-chlorothiazol-5-yl)methyl)(methyl)amino)benzisothiazole 1,1-dioxide (Compound 1)
[0194]
[0195] The first step reaction: 3-chlorobenzisothiazole 1,1-dioxide
[0196] At room temperature, 36.6g (0.20mol) of benzisothiazol-3(2H)-one 1,1 dioxide and 71.4g (0.6mol) of thionyl chloride were successively dissolved in 100ml of dioxane and refluxed for 12 Hours, TLC plate monitoring, a small amount of raw material remained. The solvent was removed under reduced pressure, and the residue was recrystallized with 60ml of toluene, filtered, and dried to obtain 30g of white solid, yield: 75%, melting point 148-149°C.
[0197] The second step reaction: 1-(2-chlorothiazol-5-yl)-nitrogen-methylmethanamine
[0198] At room temperature, add 82g (0.8mol) of 30% methylamine aqueous solution into 120ml of 95% ethanol, and cool to 0°C. 34g (0.2mol) 2-chloro-5-chloromethylthiazole was added dropwise to the above solution, the temperature was controlled below 5°C, th...
Embodiment 2
[0202] Example 2: 3-(((2-chlorothiazol-5-yl)methyl)(methyl)amino)benzisothiazole 1,1-dioxide (Compound 16)
[0203]
[0204] The first step reaction: N-((2-chlorothiazol-5-yl)-methyl)ethylamine
[0205] At room temperature, add 13.8g (0.2mol) of 65% ethylamine aqueous solution into 50ml of 95% ethanol, and cool to 0°C. Add 8.5g (0.05mol) of 2-chloro-5-chloromethylthiazole dropwise to the above solution, the temperature is controlled below 5°C, the dropwise addition takes 1h, and after the drop is complete, continue to stir at 0°C for 1h. Gradually rose to room temperature and continued to stir for 5h. TLC detects that a small amount of raw material remains. The reaction solution was concentrated under reduced pressure, 50ml of water was added to the residue, extracted with ethyl acetate (3x30ml), the combined extracts were washed with 30ml of saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 7.5g of the pr...
Embodiment 3
[0209] Example 3: 3-(((2-chlorothiazol-5-yl)methyl)(methoxy)amino)benzisothiazole 1,1-dioxide (Compound 106)
[0210]
[0211] The first step reaction: N-((2-chlorothiazol-5-yl)-methyl)-oxygen-methylhydroxylamine
[0212] At room temperature, add 8.5g (0.1mol) of methoxyamine hydrochloride and 22g (0.21mol) of triethylamine into 80ml of acetonitrile, and cool to 0°C. 16.8g (0.1mol) 2-chloro-5-chloromethylthiazole was added dropwise to the above solution, the temperature was controlled below 5°C, and the dropwise addition took 1.5h. After the dropping was completed, it was gradually raised to room temperature and continued to stir for 2h. The temperature was raised to 50°C and stirring was continued for 4h. A small amount of raw material was detected by TLC. The reaction solution was lowered to room temperature, concentrated under reduced pressure, 50ml of water was added to the residue, extracted with ethyl acetate (3x50ml), the combined extracts were washed with 50ml of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com