Method for synthesizing axitinib
A technology of axitinib and coupling reagents, which is applied in the field of synthesizing axitinib, can solve the problems of production environment pollution, low yield, complex synthesis operation, etc., to avoid production environment pollution, high reaction yield, easy operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
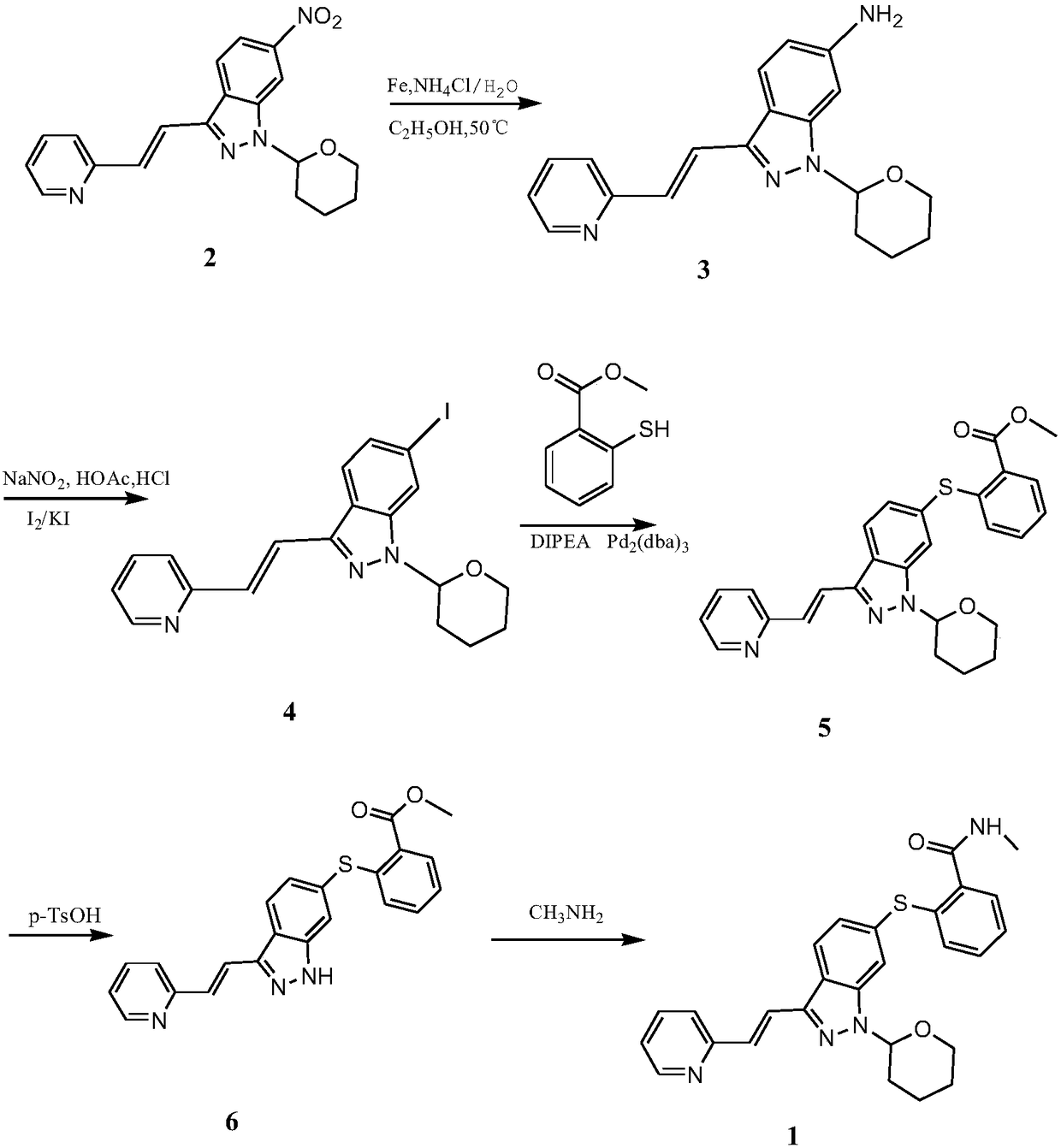
[0012] Example 1: A method for synthesizing axitinib provided by the present invention will now be described, including the following steps: (E)-6-nitro-3-[2-(2-pyridyl)vinyl]-1 -(2-Tetrahydropyranyl)-1H-indazole (2) is reduced, diazotized, Sandmeyer reaction, coupled with coupling reagent under the action of palladium catalyst, and finally deprotected and ester ammonia Solve the reaction to get Axitinib.
[0013] In this embodiment, a base is used as the reducing agent, and the base is cesium carbonate.
[0014] In this embodiment, the reaction temperature is -15°C.
[0015] In this embodiment, the coupling reagent is methyl thiosalicylate.
Embodiment 2
[0016] Example 2: Now a method for synthesizing axitinib provided by the present invention will be described, including the following steps: (E)-6-nitro-3-[2-(2-pyridyl)vinyl]-1 -(2-Tetrahydropyranyl)-1H-indazole (2) is reduced, diazotized, Sandmeyer reaction, coupled with coupling reagent under the action of palladium catalyst, and finally deprotected and ester ammonia Solve the reaction to get Axitinib.
[0017] In this embodiment, an alkali is used as the reducing agent, and the alkali is cesium hydroxide.
[0018] In this embodiment, the reaction temperature is 5°C.
[0019] In this embodiment, the coupling reagent is methyl thiosalicylate.
Embodiment 3
[0020] Example 3: The method for synthesizing axitinib provided by the present invention will now be described, including the following steps: (E)-6-nitro-3-[2-(2-pyridyl)vinyl]-1 -(2-Tetrahydropyranyl)-1H-indazole (2) is reduced, diazotized, Sandmeyer reaction, coupled with coupling reagent under the action of palladium catalyst, and finally deprotected and ester ammonia Solve the reaction to get Axitinib.
[0021] In this embodiment, a base is used as the reducing agent, and the base is diisopropylethylamine.
[0022] In this embodiment, the reaction temperature is 10°C.
[0023] In this embodiment, the coupling reagent is methyl thiosalicylate.
[0024] The present invention can also combine at least one of the technical features described in Examples 2 and 3 with Example 1 to form a new implementation.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap