Engineered immune cell capable of inducing secretion of anti-CD47 antibody
An immune cell and engineering technology, applied in the field of tumor immune cell therapy, can solve problems such as off-target toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Example 1 CAR structure and iCD47scFv structure design and transduction
[0217] 1.1 CAR structure design targeting CD19 (CD19CAR for short)
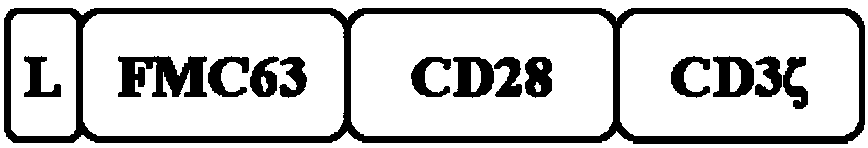
[0218] CD19CAR structure: use the second-generation CD19 CARs, including the scFv from FMC63, the hinge and transmembrane region from CD28, the intracellular segment is CD28 and CD3ζ, the structural diagram is as follows figure 1 Shown, the amino acid sequence is shown in SEQ ID NO.:1.
[0219] MLLLVTSLLL CELPHPAFLL IPDIQMTQTT SSLSASLGDR VTISCRASQD ISKYLNWYQQKPDGTVKLLI YHTSRLHSGV PSRFSGSGSG TDYSLTISNL EQEDIATYFC QQGNTLPYTF GGGTKLEITGSTSGSGKPGS GEGSTKGEVK LQESGPGLVA PSQSLSVTCT VSGVSLPDYG VSWIRQPPRK GLEWLGVIWGSETTYYNSAL KSRLTIIKDN SKSQVFLKMN SLQTDDTAIY YCAKHYYYGG SYAMDYWGQG TSVTVSSAAAIEVMYPPPYL DNEKSNGTII HVKGKHLCPS PLFPGPSKPF WVLVVVGGVL ACYSLLVTVA FIIFWVRSKRSRLLHSDYMN MTPRRPGPTR KHYQPYAPPR DFAAYRSRVK FSRSADAPAY QQGQNQLYNE LNLGRREEYDVLDKRRGRDP EMGGKPRRKN PQEGLYNELQ KDKMAEAYSE IGMKGERRRG KGHDGLYQGL STATKDTYDALHMQALPPR(SEQ ID NO.:1) ...
Embodiment 2
[0230] Example 2 Preparation of CAR-T cells
[0231]Three days after activation, the isolated and purified primary T cells were infected with lentiviral expression vectors containing CD19-CAR and CD19-CAR-iCD47scFv, transferred to cell culture flasks, and placed at 37°C, 5% CO 2 cultured in a constant temperature incubator. On the 3rd and 7th day after infection, Protein L (Thermo Fisher Scientific) was used to detect the CAR positive rate of T cells, and half of the medium was replaced every 2-3 days. The CAR-T cells obtained after culture were CD19 CAR-T cells and iCD47scFv-CD19 CAR-T cells.
Embodiment 3
[0232] Example 3 CAR-T cytokine release detection
[0233] CD19 CAR-T cells and iCD47scFv-CD19 CAR-T cells obtained in Example 2 were combined with tumor cells (CD19 + K562) were mixed at a ratio of 1:1, and the control group was CD19 CAR-T cells and iCD47scFv-CD19 CAR-T cells were mixed with K562 cells at a ratio of 1:1. 6 / ml, 100ul each of CAR-T cells and tumor cells were placed in a 96-well plate, co-cultured overnight, the supernatant was collected, centrifuged and the supernatant was taken to detect the release levels of cytokines IFN-γ, IL-2 and CD47 antibodies, Elisa kit (Biolegend) was used for detection.
[0234] The results showed that the release of IFN-γ and IL-2 in CD19 CAR-T cells and iCD47scFv-CD19 CAR-T cell groups were significantly higher than those in the control group. The expression of CD47 scFv could only be detected in the experimental group activated by iCD47scFv-CD19 CAR-T cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com