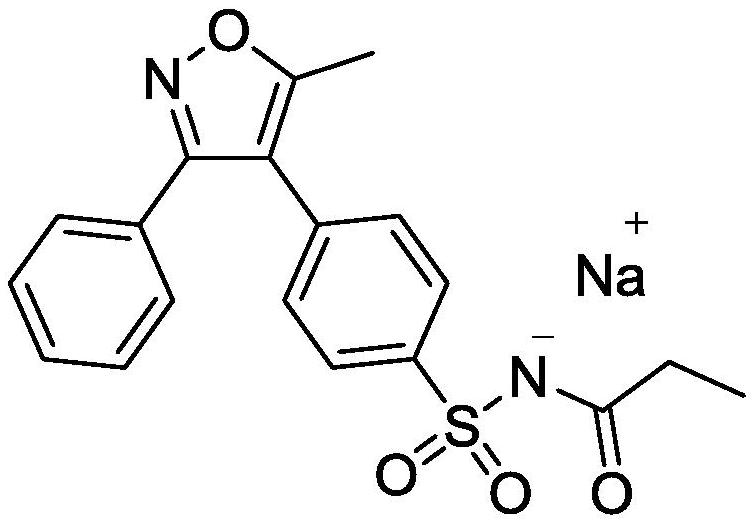
Application of citric acid in the preparation of parecoxib sodium freeze-dried preparation composition and its composition and preparation method
A technology of parecoxib sodium and freeze-dried preparations, which can be applied in the directions of drug combination, freeze-dried delivery, active ingredients of heterocyclic compounds, etc. The impact of product quality, product quality and clinical drug delivery risks, etc., to achieve the effect of improving clinical drug delivery safety and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
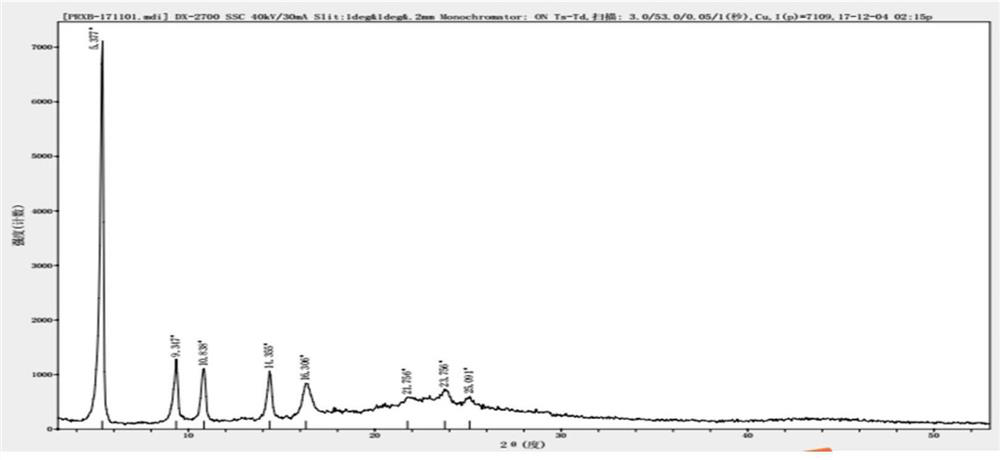
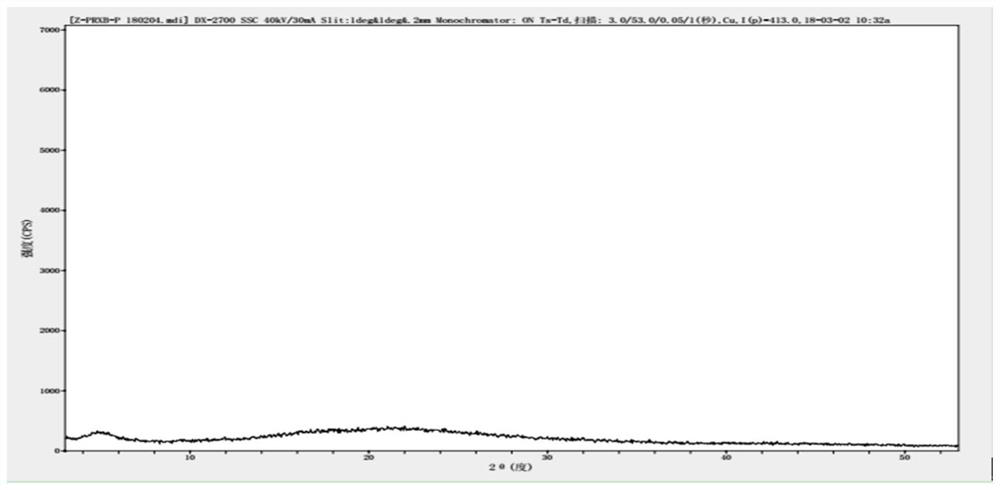
[0058] The application of citric acid as a light stabilizer in the preparation of parecoxib sodium freeze-dried preparation composition to prevent or delay the exposure of parecoxib sodium freeze-dried preparation composition to light source in production, storage, transportation and clinical use Impurities U and T are produced under these conditions.
[0059] In the present embodiment, the 20mg specification formula is as follows:
[0060]
[0061] In the present invention, the parecoxib sodium freeze-dried preparation containing the pharmaceutical light stabilizer is prepared by the following method:
[0062] (1) take by weighing the water for injection of 95% ± 2% of the full amount of the production recipe in the preparation tank, with the anhydrous disodium hydrogen phosphate and citric acid monohydrate of the recipe amount, after opening stirring and dissolving; then add the Parry of the recipe amount Coxib sodium, stir to dissolve completely, mix well, cool the liqu...
Embodiment 2
[0079] 40mg specification formula
[0080]
[0081] The preparation method of the parecoxib sodium freeze-dried preparation containing the pharmaceutical light stabilizer in the present invention is shown in Example 1; the filling amount is 2ml of medicinal liquid into a 7ml medium borosilicate vial.
Embodiment 3
[0082] Embodiment 3: 20mg specification formula
[0083]
[0084]
[0085] The preparation method of the freeze-dried preparation of parecoxib sodium containing a pharmaceutical light stabilizer in the present invention is shown in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com