Combined drug and application thereof in preparing drugs for treating high-level cerebroma with postoperative recurrence and no response to standard treatment
A high-level, drug-based technology, applied in drug delivery, drug combination, medical preparations containing active ingredients, etc., can solve the problem of no chemotherapy program and achieve the effect of prolonging survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Combination drugs of the present invention
[0031] 1Chlorogenic acid injection
[0032] Weigh 350g of chlorogenic acid aseptically, add 850g of mannitol, and dispense into injections after preparation.
[0033] 2 Temozolomide capsules
[0034] Weigh 100 g of temozolomide aseptically, and pack them into capsules aseptically.
[0035] 3 how to use
[0036] First orally use temozolomide, and then continue to use chlorogenic acid injection according to the mass ratio of 300 (chlorogenic acid): 150 (temozolomide).
Embodiment 2
[0037] Example 2 Combination drugs of the present invention
[0038] 1 Chlorogenic acid oral tablet
[0039] Weigh 350g of chlorogenic acid aseptically, add 850g of mannitol and 250g of microcrystalline cellulose, and make oral tablets after compression.
[0040] 2 Temozolomide capsules
[0041] Weigh 100 g of temozolomide aseptically, and pack them into capsules aseptically.
[0042] 3 how to use
[0043] First orally use temozolomide, and then continue to use chlorogenic acid tablets according to the mass ratio of 300 (chlorogenic acid): 150 (temozolomide).
Embodiment 3
[0044] Example 3 Combination drugs of the present invention
[0045] 1Chlorogenic acid injection
[0046] Weigh 100g of chlorogenic acid aseptically, add 650g of mannitol, and dispense into injections after preparation.
[0047] 2 Temozolomide capsules
[0048] Weigh 100 g of temozolomide aseptically, and pack them into capsules aseptically.
[0049] 3 how to use
[0050] According to the mass ratio of 100 (chlorogenic acid): 100 (temozolomide), both temozolomide capsules and chlorogenic acid injection were used.
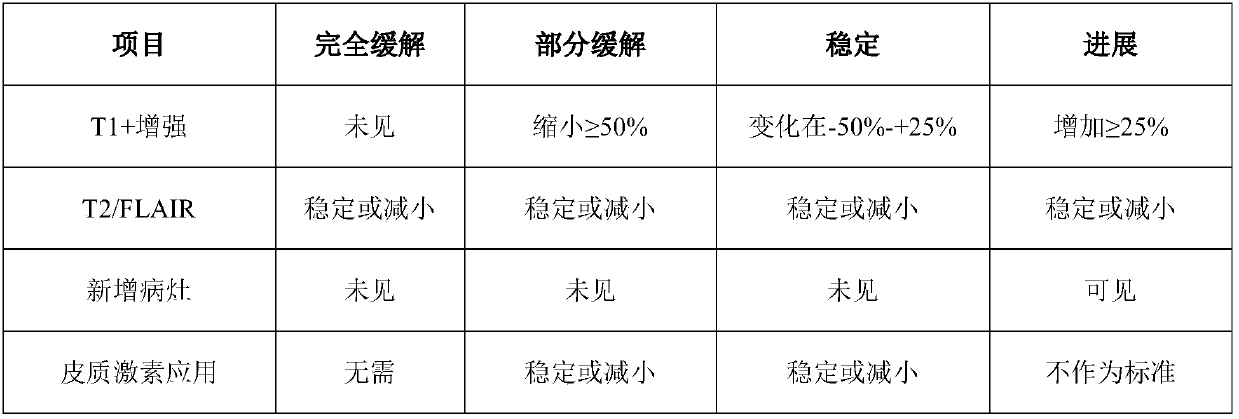
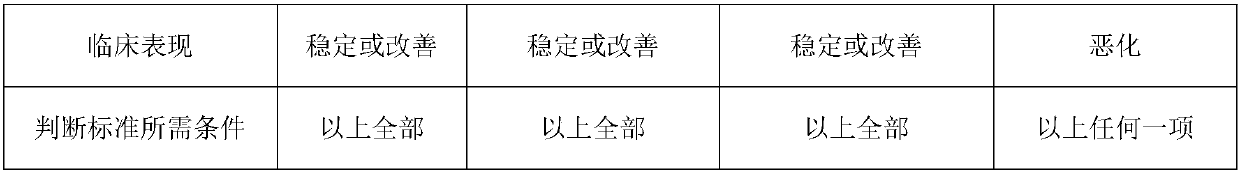
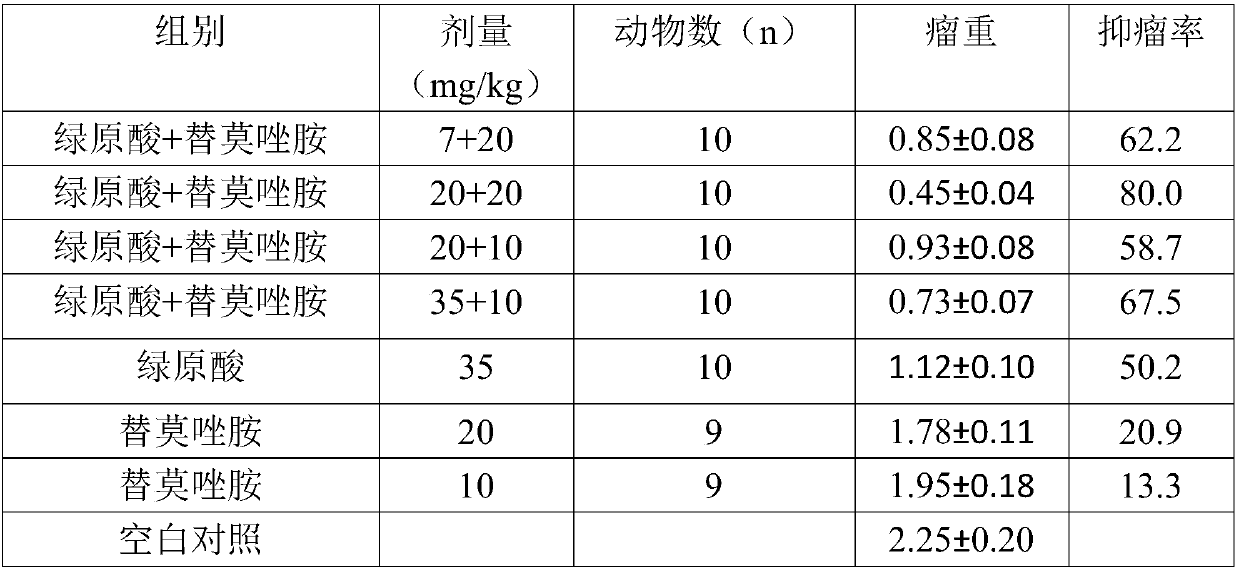
[0051] 4 Animal model: Temozolomide resistance animal model
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com