Dendrimers and their synthetic methods and applications
A synthesis method and dendritic technology are applied in the field of dendrimer-like polymers and their synthesis, and can solve the problems of complex steps, low yield of target polymers, low activity of grafting sites and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
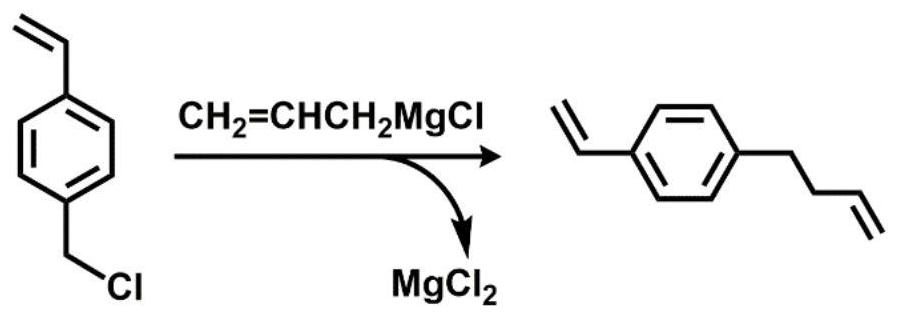
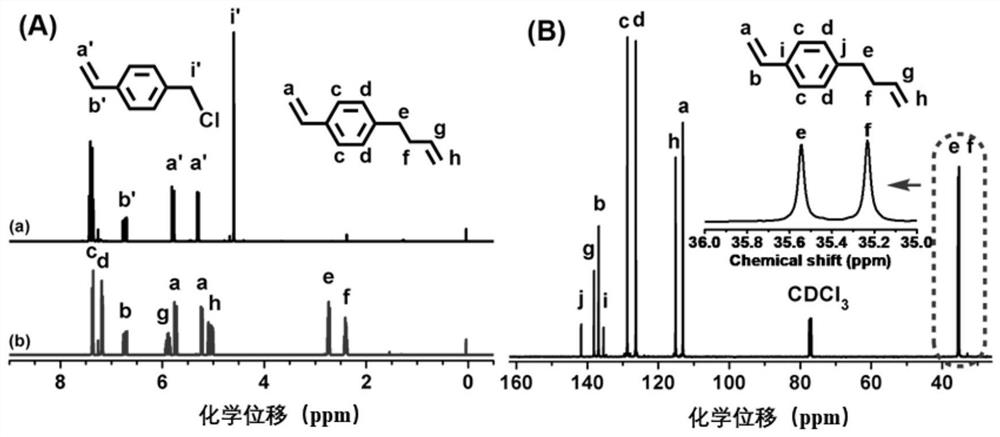
[0034] Embodiment 1: synthetic VSt
[0035] VSt can be generated by coupling 4-chloromethylstyrene (CMS) with allylmagnesium chloride. Under the protection of argon, add 200 mL THF solution (1.0 mol / L) of allylmagnesium bromide into the four-neck flask equipped with a constant pressure dropping funnel, and then add 23 mL CMS (0.16 mol) and 50 mL THF. After the reaction system was cooled to 0° C. with ice-water mixture, the THF solution of CMS was slowly dropped into the flask, the reaction system was raised to room temperature, and magnetically stirred for 12 h under the protection of argon. After the reaction, slowly add 20 mL of saturated ammonium chloride (NH 4 Cl) solution. The reaction solution was poured into a separatory funnel, the product was extracted with ether, and saturated NH 4 Cl solution was washed three times. Finally, put VSt and appropriate amount of CaH 2 Add it into a round bottom flask, stir at room temperature for 12 h, then distill it off with a v...
Embodiment 2
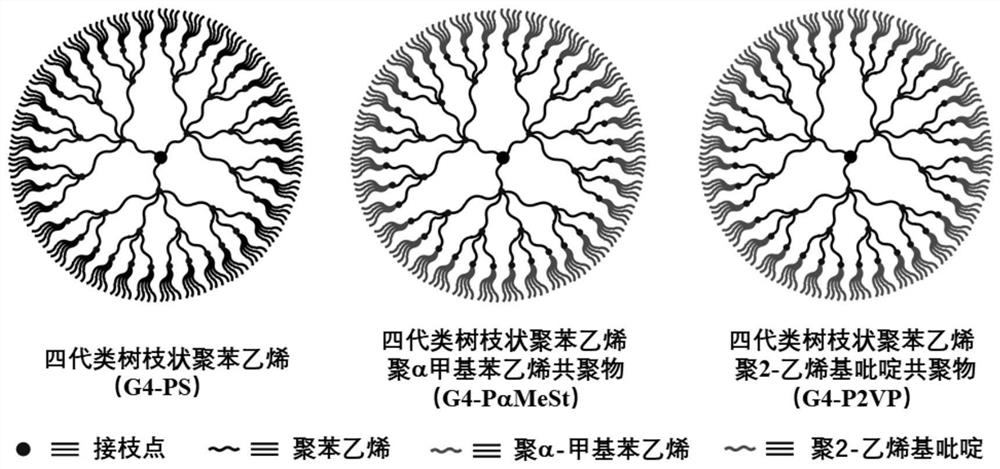
[0036] Embodiment 2: Synthesis of three generations of dendrimers (G3- g -SiCl)
[0037] In a vacuum system, use 100 mL of n -BuLi decontaminated toluene and 50 mL n -BuLi-purified THF was flash evaporated into the anion reaction vial. Fill the reaction bottle with dry argon until the pressure in the bottle is slightly higher than atmospheric pressure, and add 2.8 mL VSt (15.9 mmol) into the bottle with a syringe, cool the reaction solution to -45°C with an acetonitrile / liquid nitrogen bath, and then add to the reaction bottle. Add 1.5 mL to the solution s -BuLi in n-hexane solution, the reaction solution immediately turns orange. Under the condition of -45°C, magnetically stir for 30 minutes to obtain the active anion chain PVStLi. Add 16.8 mL of styrene (146.2 mmol) that had been removed with dibutylmagnesium to the reaction solution, and the color of the reaction solution darkened slightly. - At 45°C, magnetically stirred for 1 h to obtain the active anion chain PVSt-...
Embodiment 3
[0040] Example 3: Synthesis of four generations of dendritic polystyrene (G4-PS)
[0041] In a vacuum system, the n - The THF from which BuLi was removed and the St from which Dibutyl Magnesium was removed were flashed into the reaction bottle, and dry argon was filled into the reaction bottle until the pressure in the bottle was slightly higher than the atmospheric pressure. After the reaction system cools down to -78°C, add s -BuLi, the solution immediately becomes orange-red. Under the condition of -78°C, magnetically stirred for 1 h to obtain the active anion chain PSLi. A small amount of reaction liquid was taken and terminated with degassed methanol, and the resulting polymer was used to analyze the degree of polymerization of grafted PS. Add G3- to the THF solution of PSLi g-SiCl in toluene solution, and gradually warmed to -45 °C, magnetically stirred for 2 h, and the excess PSLi was terminated with degassed methanol. With methanol as a poor solvent and toluene as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com