Application of Recombinant Bacillus subtilis in Prevention or Treatment of Mycoplasma Hyopneumoniae Infection
A Bacillus subtilis and carrier technology, applied in the field of biotechnology genetic engineering, can solve the problem of not preventing Mycoplasma hyopneumoniae infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
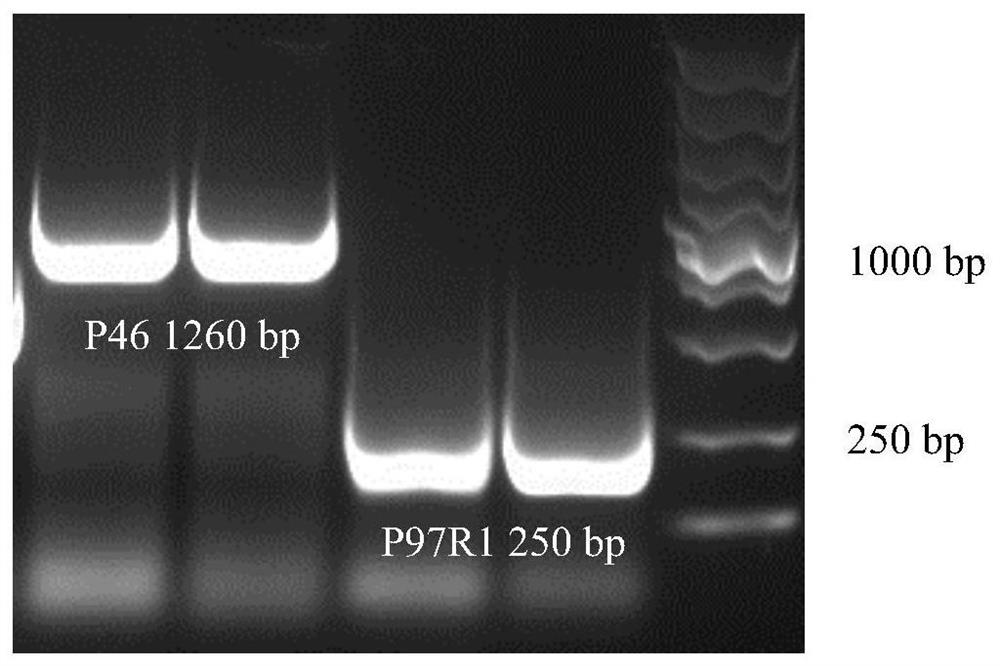
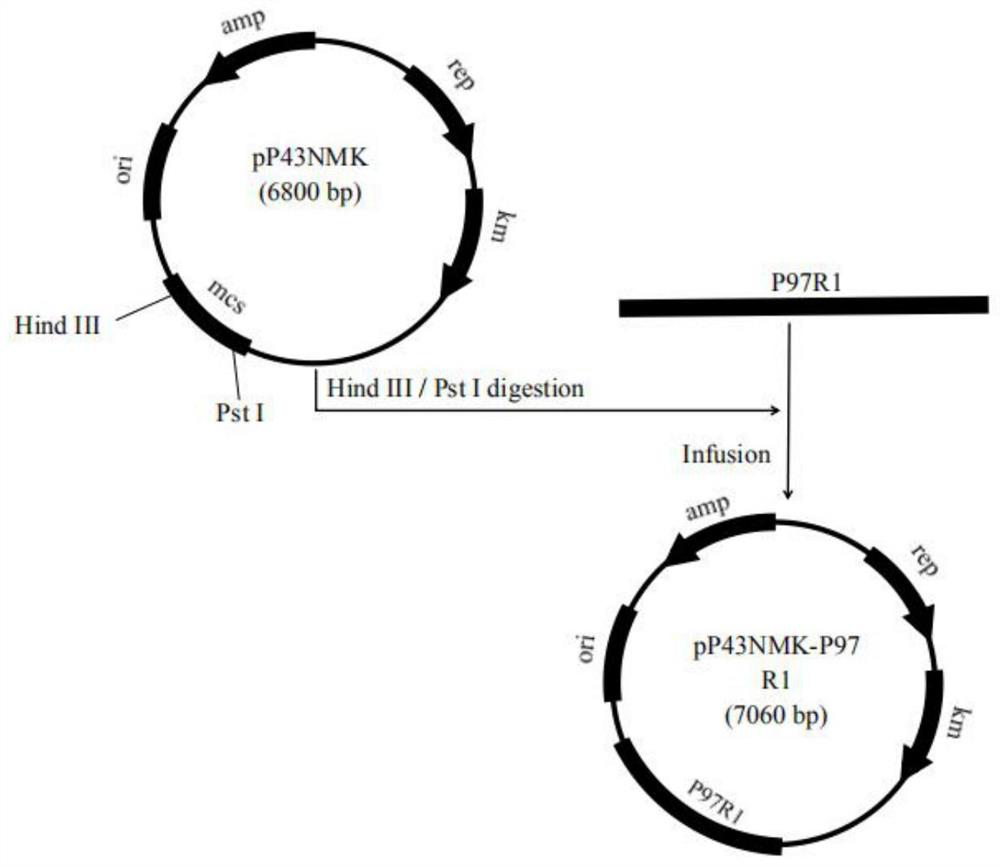
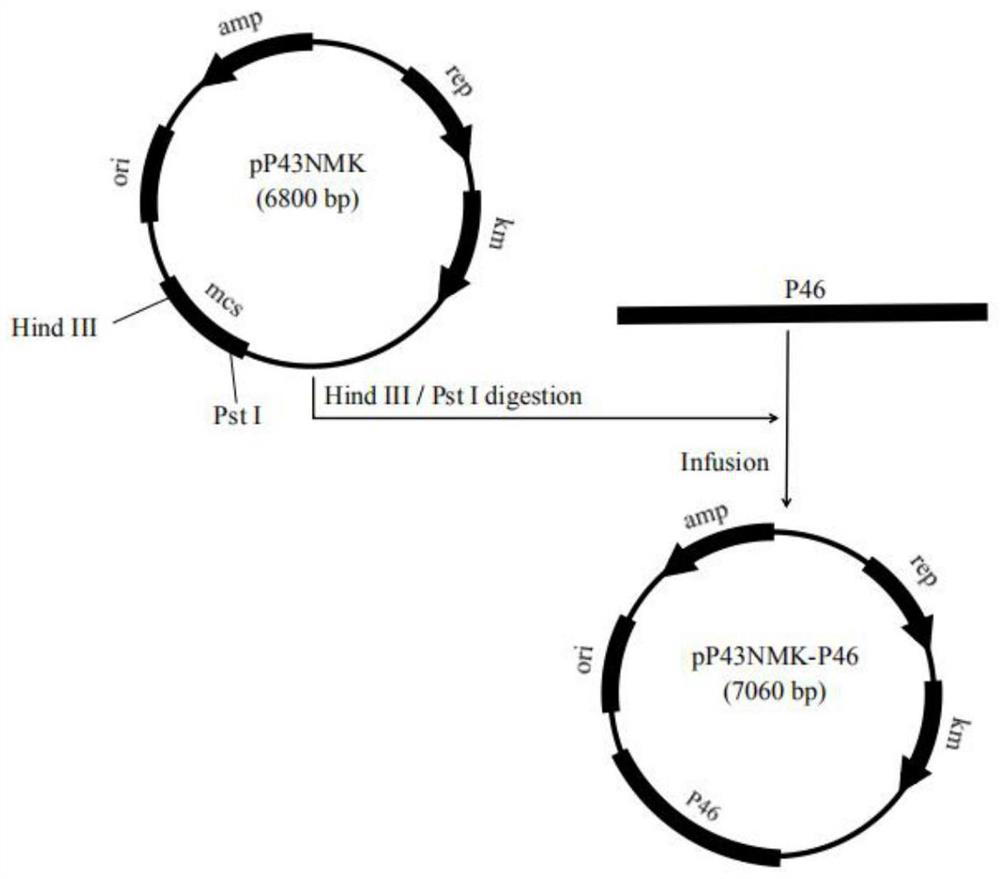
[0040] Example 1 Construction of recombinant Bacillus subtilis B.S-P97R1 and B.S-P46 expressing Mycoplasma hyopneumoniae P97R1 and P46 genes
[0041] 1. Design and synthesis of primers:
[0042] According to the P97R1 and P46 gene sequences of 168 strains of Mycoplasma hyopneumoniae published in GenBank (gifted by researcher Feng Zhixin of Jiangsu Academy of Agricultural Sciences Veterinary Research Institute) (NC_017509.1) (see SEQ ID NO: 1 and SEQ ID NO: 2 in the sequence listing), Primers were designed with the following sequences:
[0043] P97R1-F: 5'-CATTACCTCAGCCGCCAGCAG-3',
[0044] P97R1-R: 5'-AAGCCATTGGGAAATAGTCT-3';
[0045] P46-F: 5'-AAATGAAAAAAATGCTTAG-3',
[0046] P46-R: 5'-TTAGGCATCAGGATTATCAACAT-3';
[0047] The above primers were synthesized by Nanjing GenScript Biotechnology Company.
[0048] 2. Template preparation:
[0049] Take 168 cultured Mycoplasma hyopneumoniae strains, and use the boiling method to roughly extract the whole genome. The specific o...
Embodiment 2
[0078] Example 2 Test of Recombinant Bacillus subtilis B.S-P97R1, B.S-P46 Nasal Spray Immunization on Mice
[0079] 1. Select 80 6-week-old SPF female mice (Qinglongshan Animal Breeding Farm, Jiangning District, Nanjing City), and randomly divide them into 4 groups, 20 in each group. Group 1: blank control group (PBS group, same dose of 20 μL sterile PBS nasal drop mice); group 2: empty vector group (B.S group, same dose (20μL 5×10 8CFU B.S) nasal drop mice); group 3: B.S-P97R1 group (on the 0th day nasal drop immunization 20μL 5×10 8 CFU B.S-P97R1, second immunization with the same dose of bacteria on the 28th day); group 4: B.S-P46 group (20μL 5×10 nasal drop immunization on the 0th day 8 CFU B.S-P46, second immunization with the same dose of bacteria on the 28th day), and on the 0th day, 7th day, 14th day, and 28th day after immunization, 4 animals in each group were killed to collect serum and alveolar washing fluid, and on the 42nd day all They were sacrificed, and seru...
Embodiment 3
[0082] Example 3 Recombinant Bacillus subtilis B.S-P97R1, B.S-P46 nasal spray immunization test of 21-day-old piglets
[0083] 1. Select 6 21-day-old SPF piglets (Veterinary Research Institute of Jiangsu Academy of Agricultural Sciences), and randomly divide them into 2 groups, 3 piglets in each group. The immune group (immunized and challenged) was first immunized on the 0th day (1mL 5× 10 9 CFU B.S-P97R1 mixed 1mL 5×10 9 CFU B.S-P46 nasal spray immunization), 28 days of secondary immunization (immunization with the same dose); the control group (not immunized but challenged) with 2 mL sterile PBS nasal spray control. Serum was collected 14 days, 28 days, and 42 days after immunization (aseptically collect blood from the anterior vena cava into an anticoagulant tube, let it stand overnight at 4°C, centrifuge at 2500r / min at 4°C for 10min, collect the supernatant as a test sample, and store it at -20 ℃ frozen for later use) and nasal swabs (take the two nostril swabs of pigs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com