Clinical testing case report table quality control system and quality control method
A case report form and clinical trial technology, applied in the quality control system and quality control field of clinical trial case report form, can solve the problems of low degree of informatization and low quality control efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
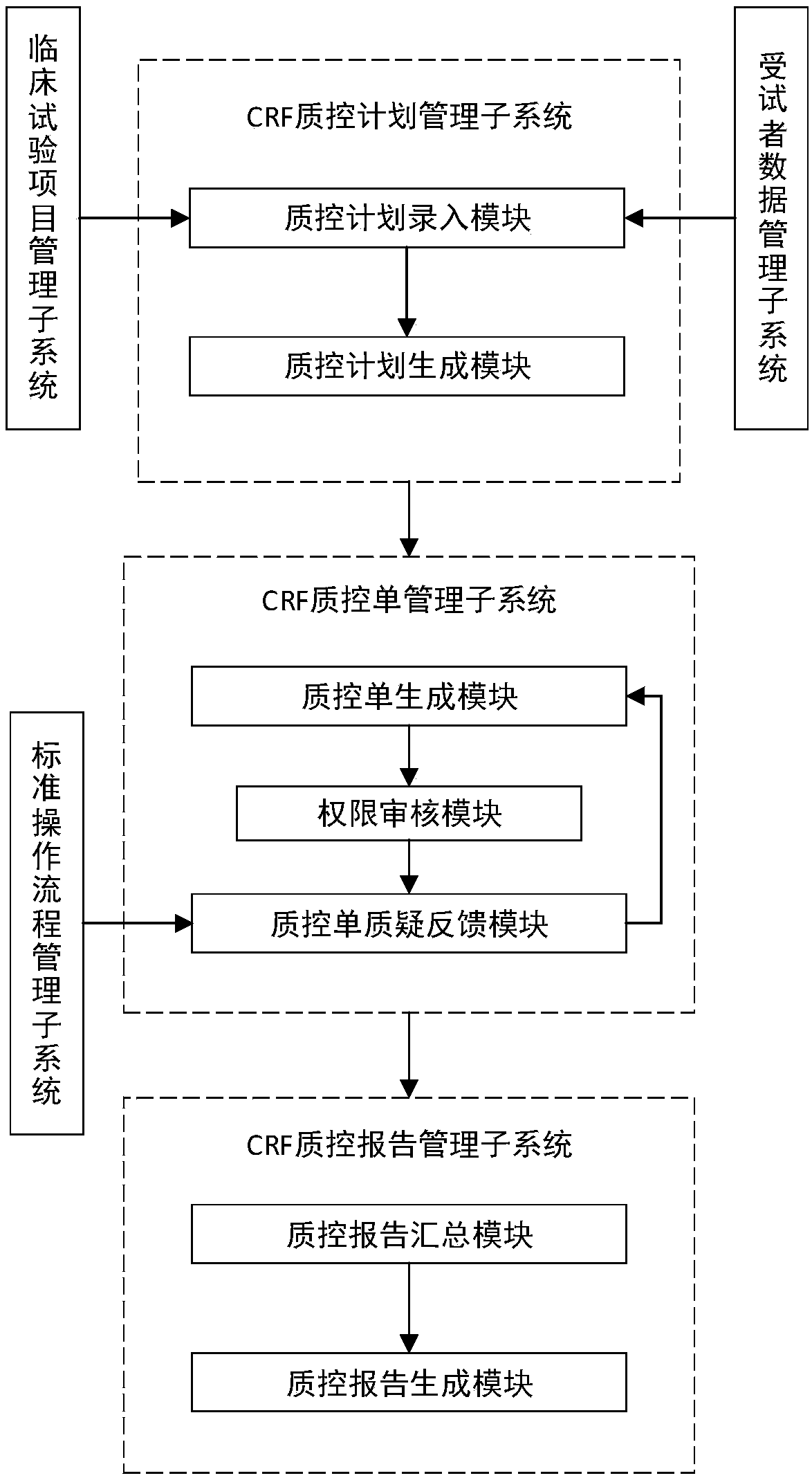
[0038] see figure 1 , a schematic structural diagram of a quality control system for a clinical trial case report form provided by an embodiment of the present invention. The embodiment of the present invention provides a quality control system for clinical trial case report form, including: CRF quality control plan management subsystem, CRF quality control sheet management subsystem and CRF quality control report management subsystem, wherein, CRF quality control plan management subsystem The output end of the subsystem is connected with the input end of the CRF quality control sheet management subsystem, and the output end of the CRF quality control sheet management subsystem is connected with the input end of the CRF quality control report management subsystem.
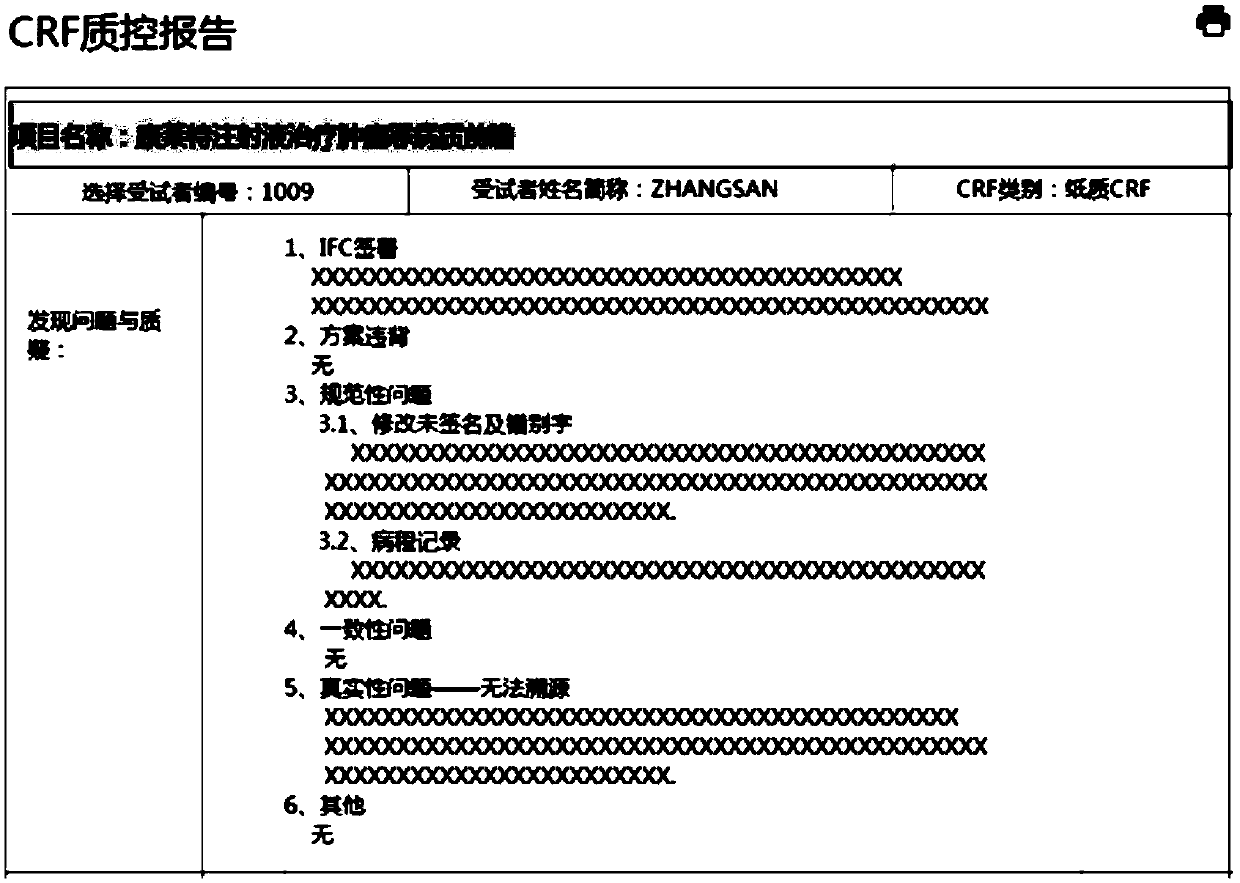
[0039] see figure 2 and image 3 , the work effect diagram and the quality control report effect diagram of the quality control report summary module in the clinical trial case report form quality control system...
Embodiment 2
[0050] see Figure 4 , the flow chart of the quality control method for the clinical trial case report form provided by the embodiment of the present invention. The embodiment of the present invention provides a kind of clinical trial case report form quality control method, comprises the following steps:
[0051](1) Read the clinical trial project information in the externally connected clinical trial project management subsystem, and load the subject data corresponding to the clinical trial project information into the subject data management subsystem. Specifically, the clinical trial project information includes the project number, project name, and project data files. Specifically, the project data files include the materials submitted during the clinical trial project application stage and the record files during the clinical trial project execution stage. In the quality control process of clinical trial projects, it is necessary to monitor the basic data and clinical d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com