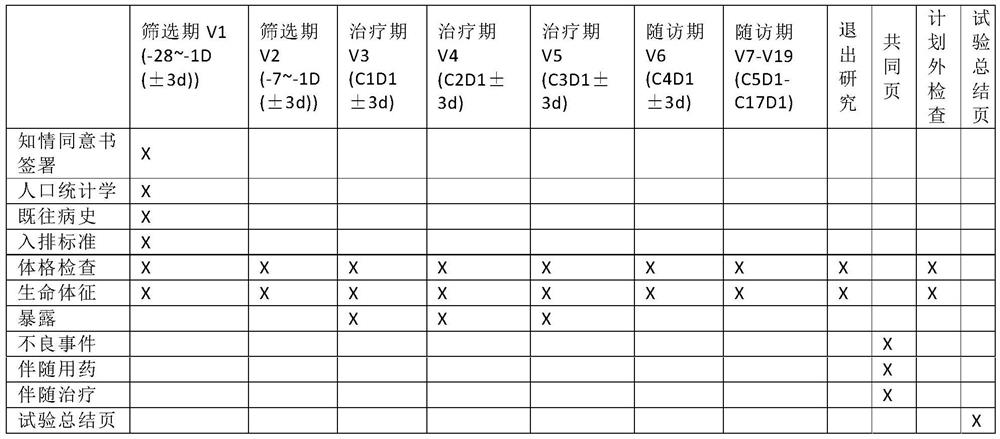
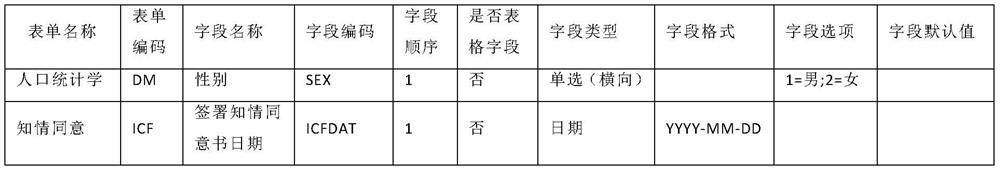
Patents
Literature
37 results about "Case report form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A case report form (or CRF) is a paper or electronic questionnaire specifically used in clinical trial research. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. All data on each patient participating in a clinical trial are held and/or documented in the CRF, including adverse events.
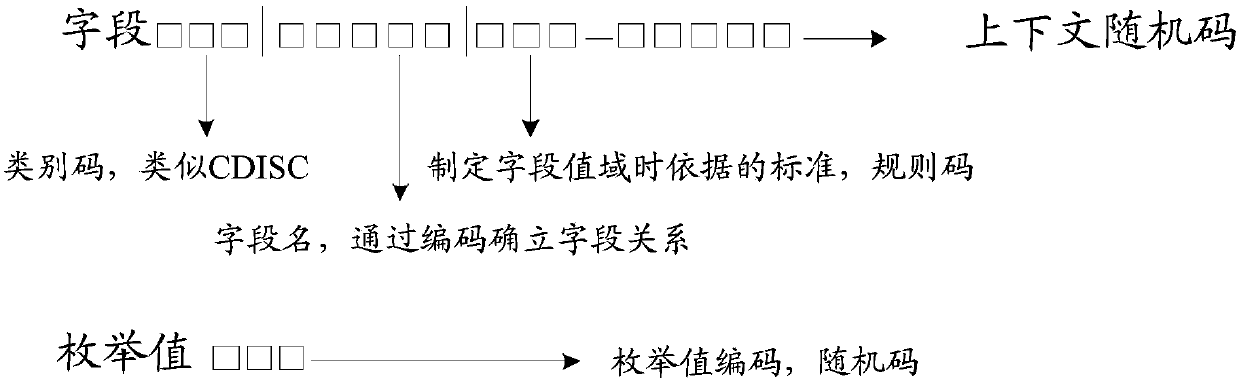
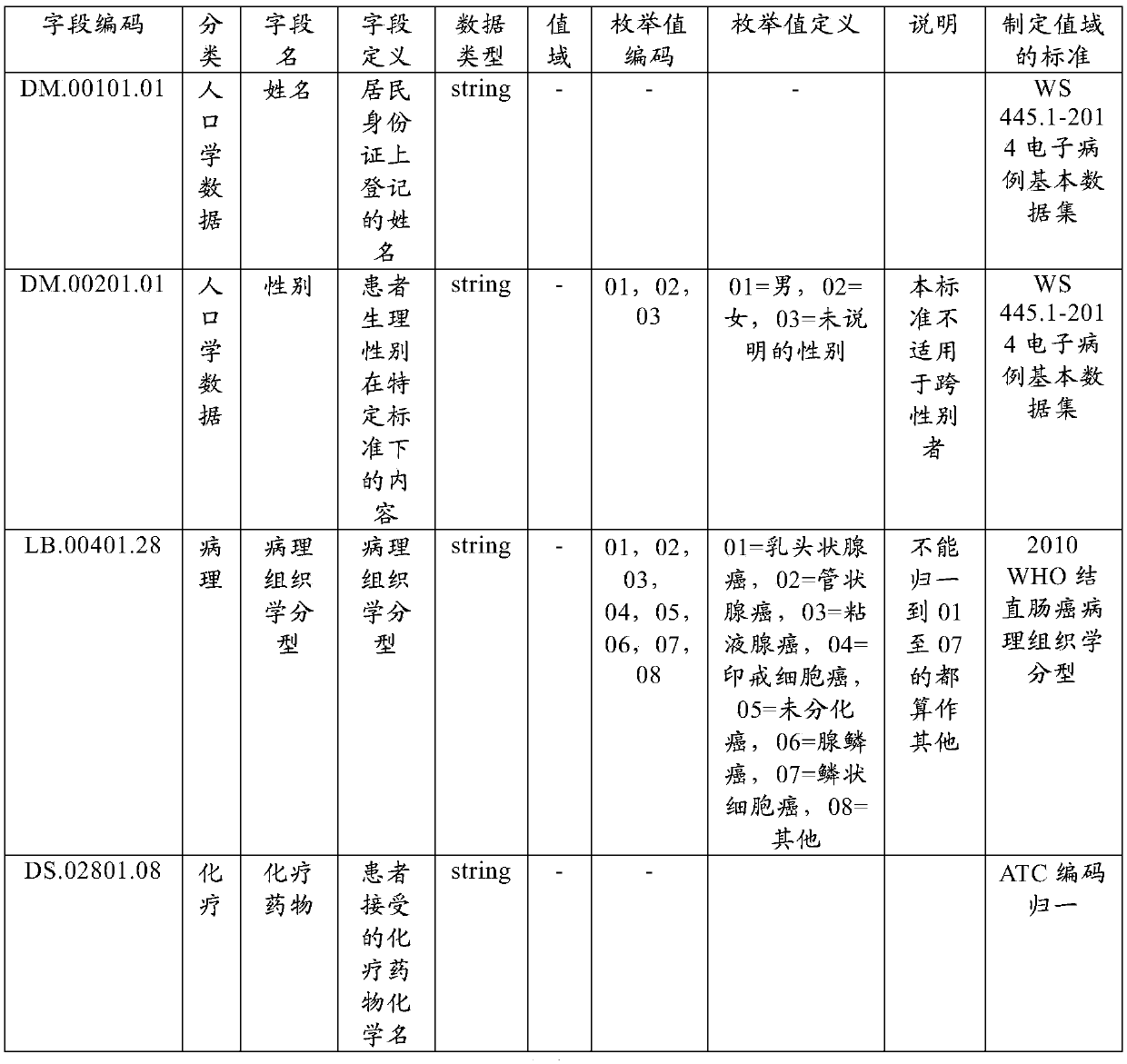
Method for optimizing clinical data standardization
InactiveUS20100299335A1Digital data information retrievalDigital data processing detailsData setClinical trial
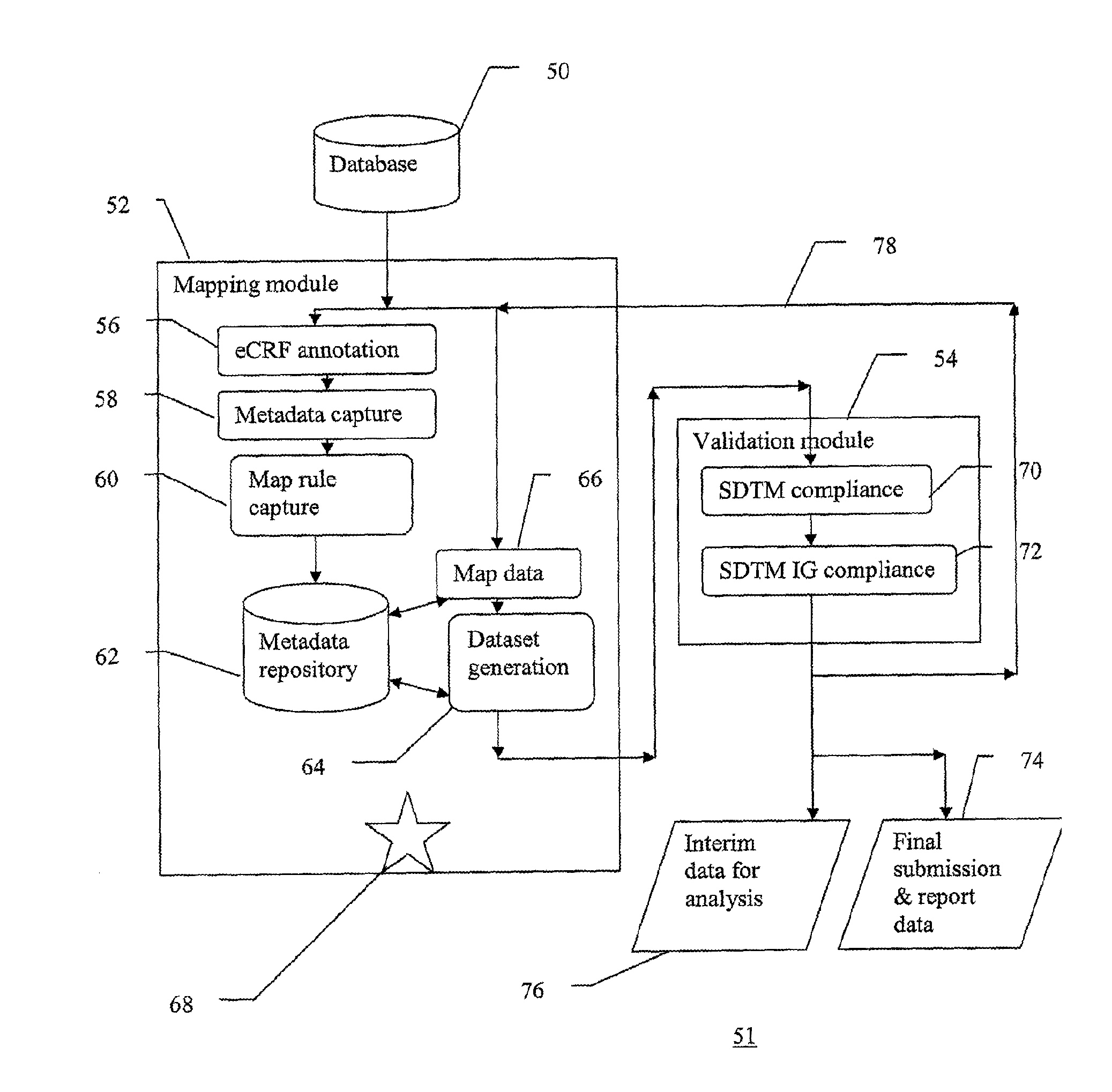
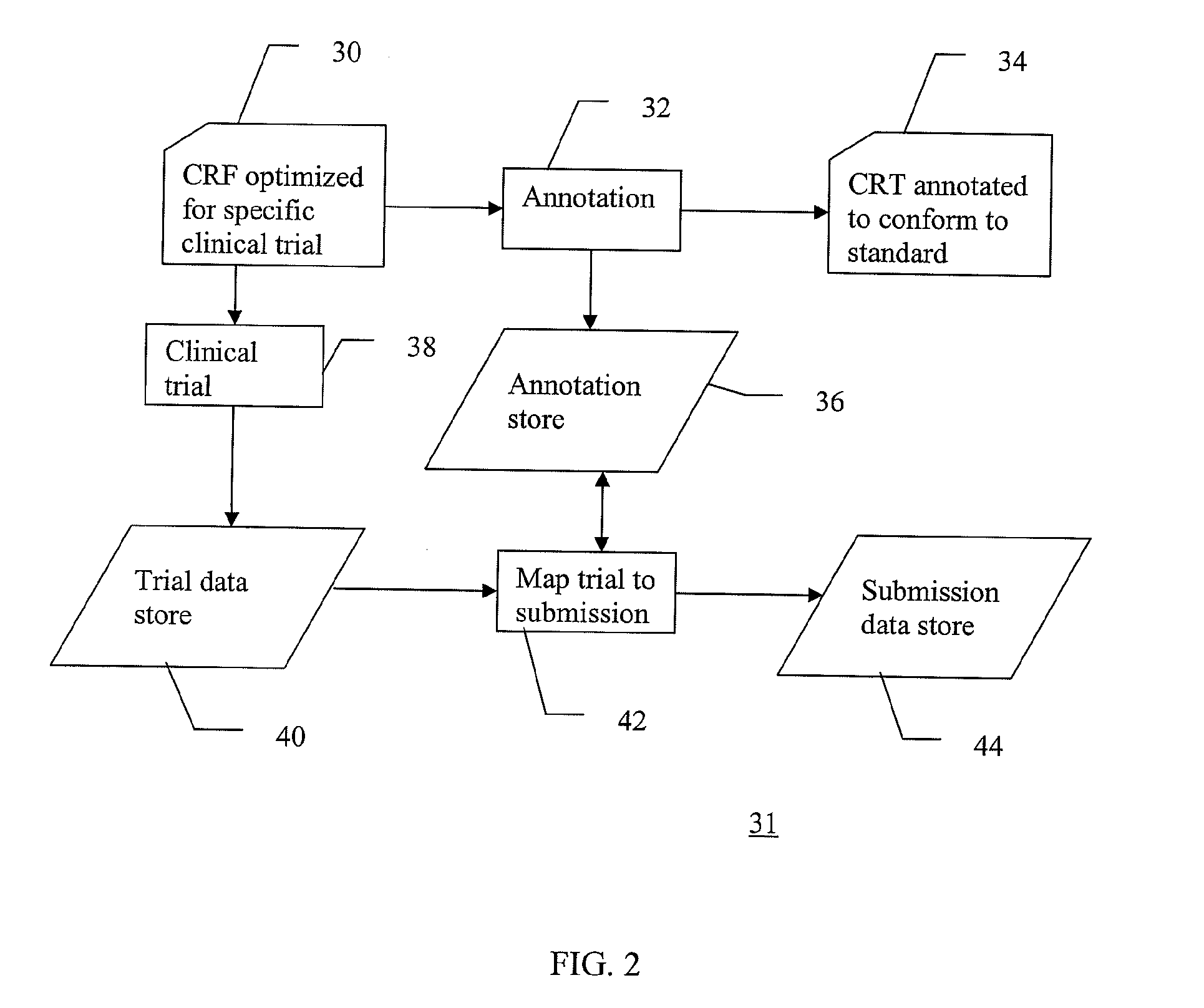
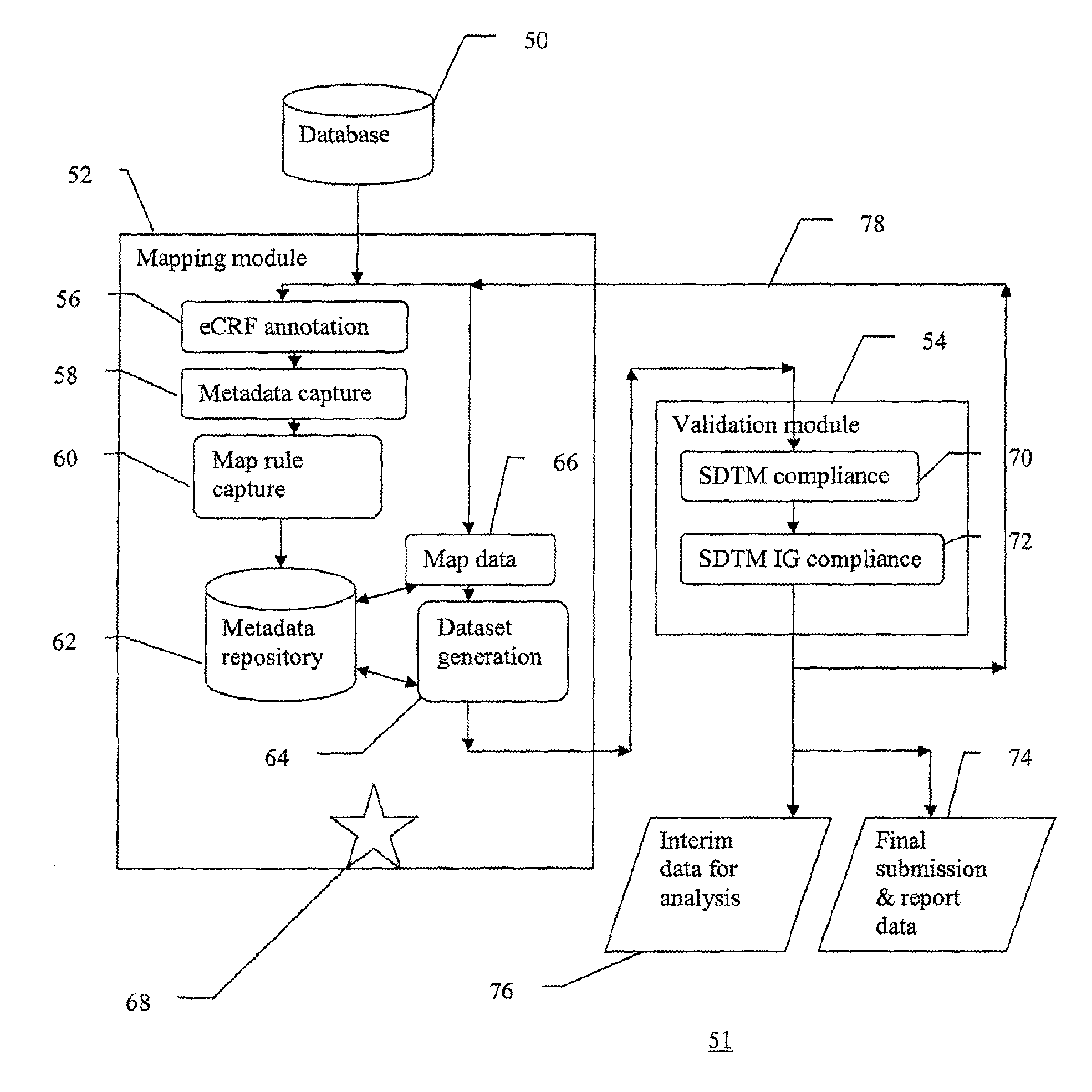
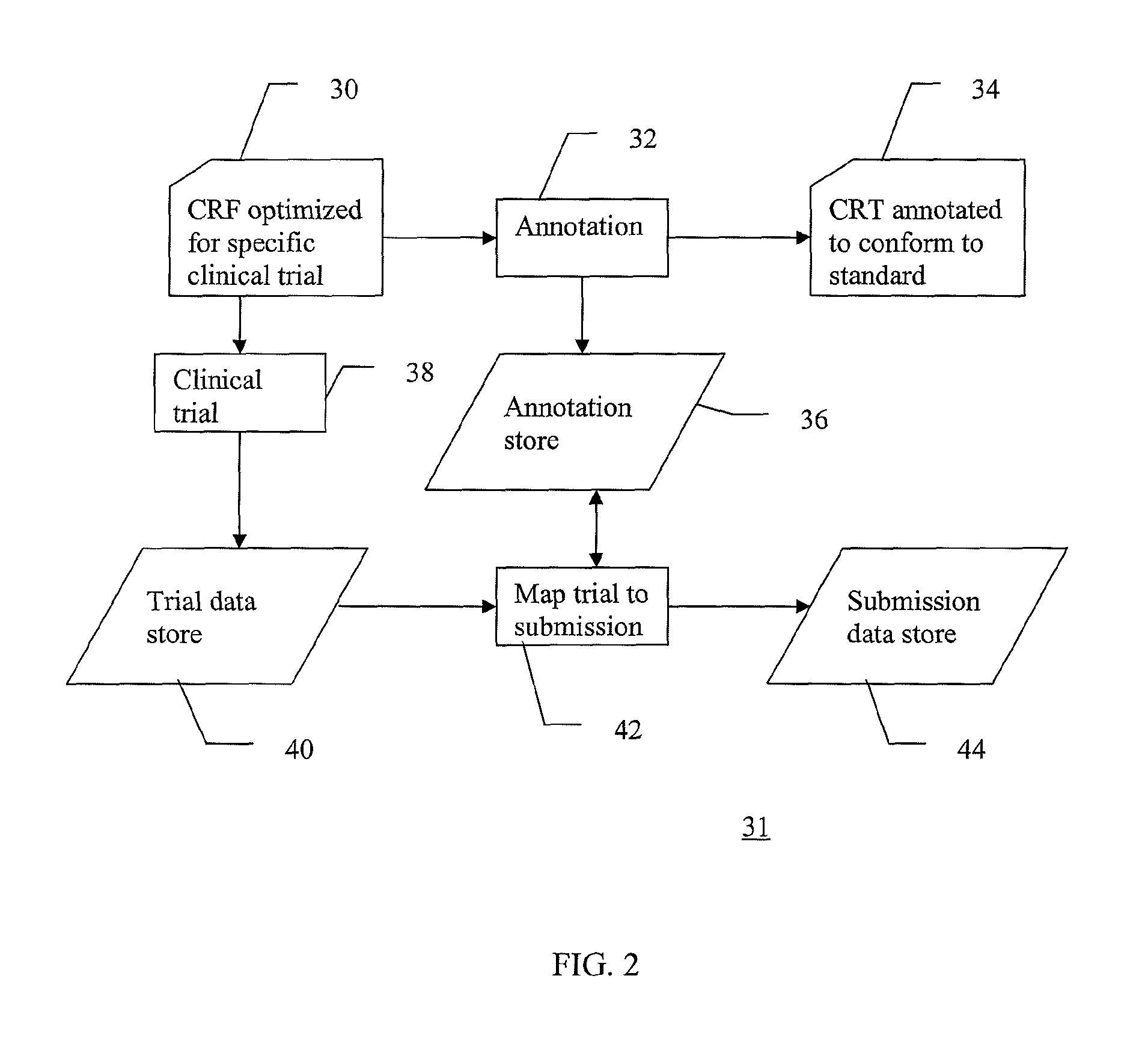
A method of integrating clinical trial data into a form required by a standards based data format. Annotations may be made to a case report form (CRF) designed for a clinical trial that map report form data to standards compliant data. The annotations may be stored in, and then applied to data captured using that particular case report form to produce standards compliant data for that particular clinical trial. The data sets produced may then be validated as being standards compliant.
Owner:GOPALAKRISHNAN KALYAN +2
Case report form fill-in method and device, electronic equipment and storage medium
PendingCN107767929ALow costImprove research efficiencyMedical reportsInstrumentsResearch efficiencyData mining
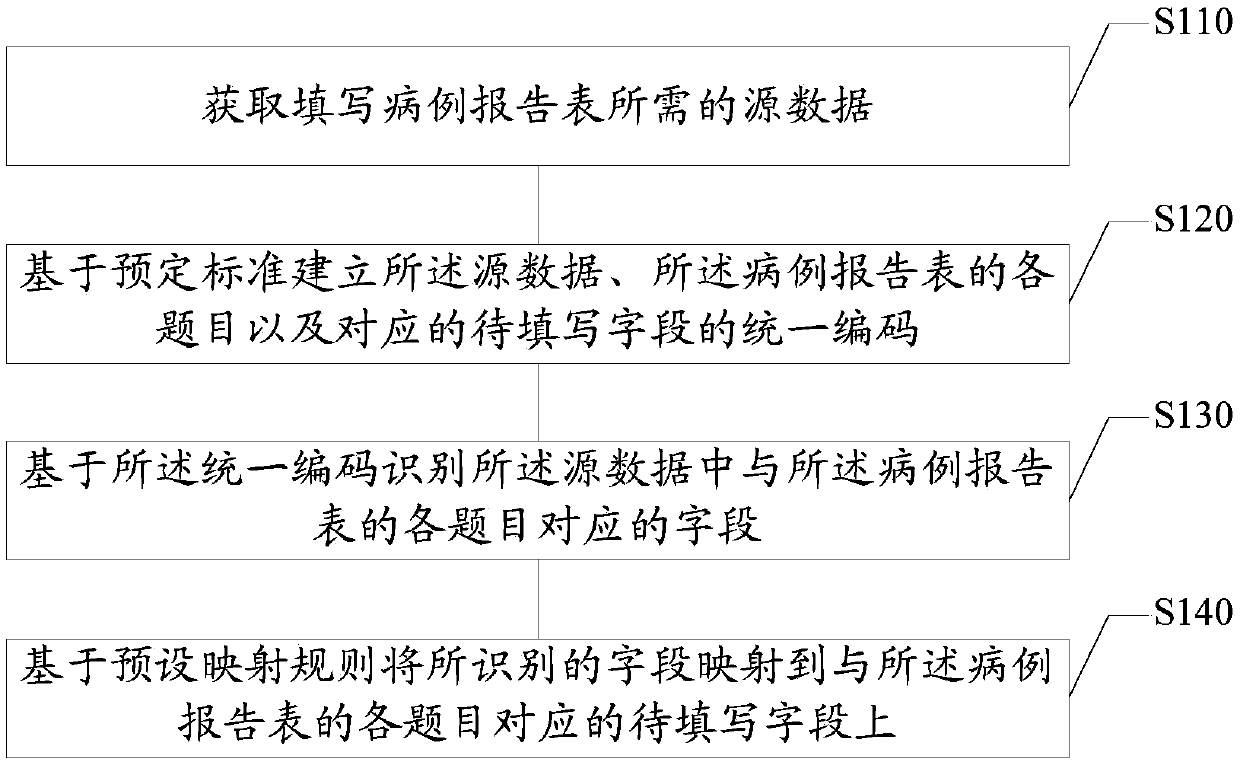
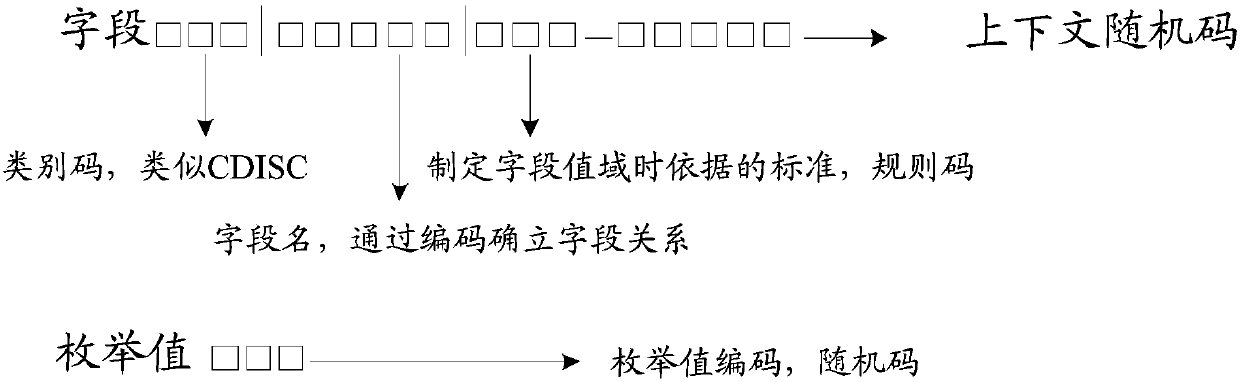
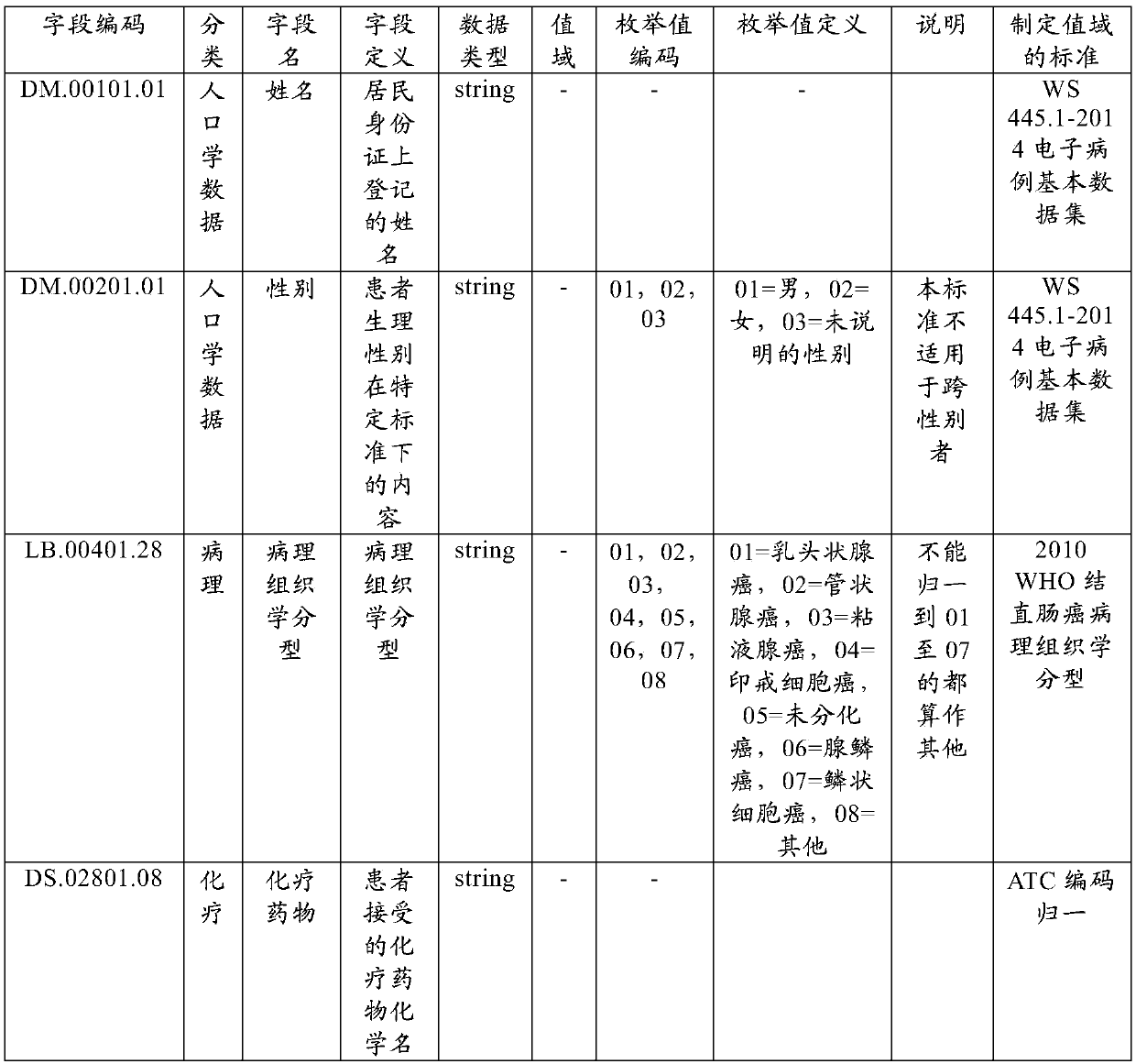
The embodiment of the invention provides a case report form fill-in method and device, electronic equipment and a storage medium, and relates to the technical field of data processing. The method comprises the steps that source data required for filling in a case report form are acquired; the source data, all the topics of the case report form and the corresponding unified codes of fields to be filled in are established based on the preset standard; the fields corresponding to all the topics of the case report form in the source data are identified based on the unified codes; and the identified fields are mapped to the corresponding fields to be filled in of all the topics of the case report form based on the preset mapping rules. According to the technical scheme, the case report form canbe automatically filled in so that the cost of the research project can be reduced and the research efficiency can be enhanced.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
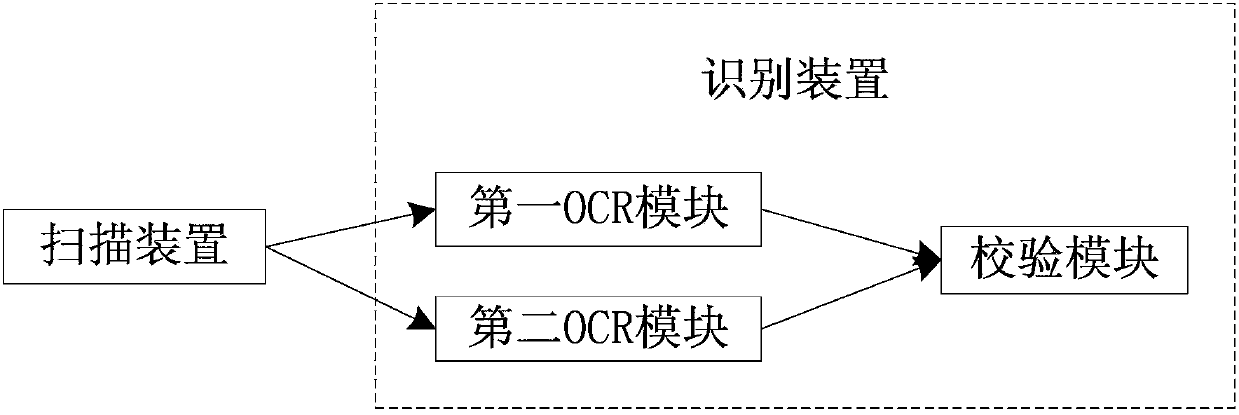
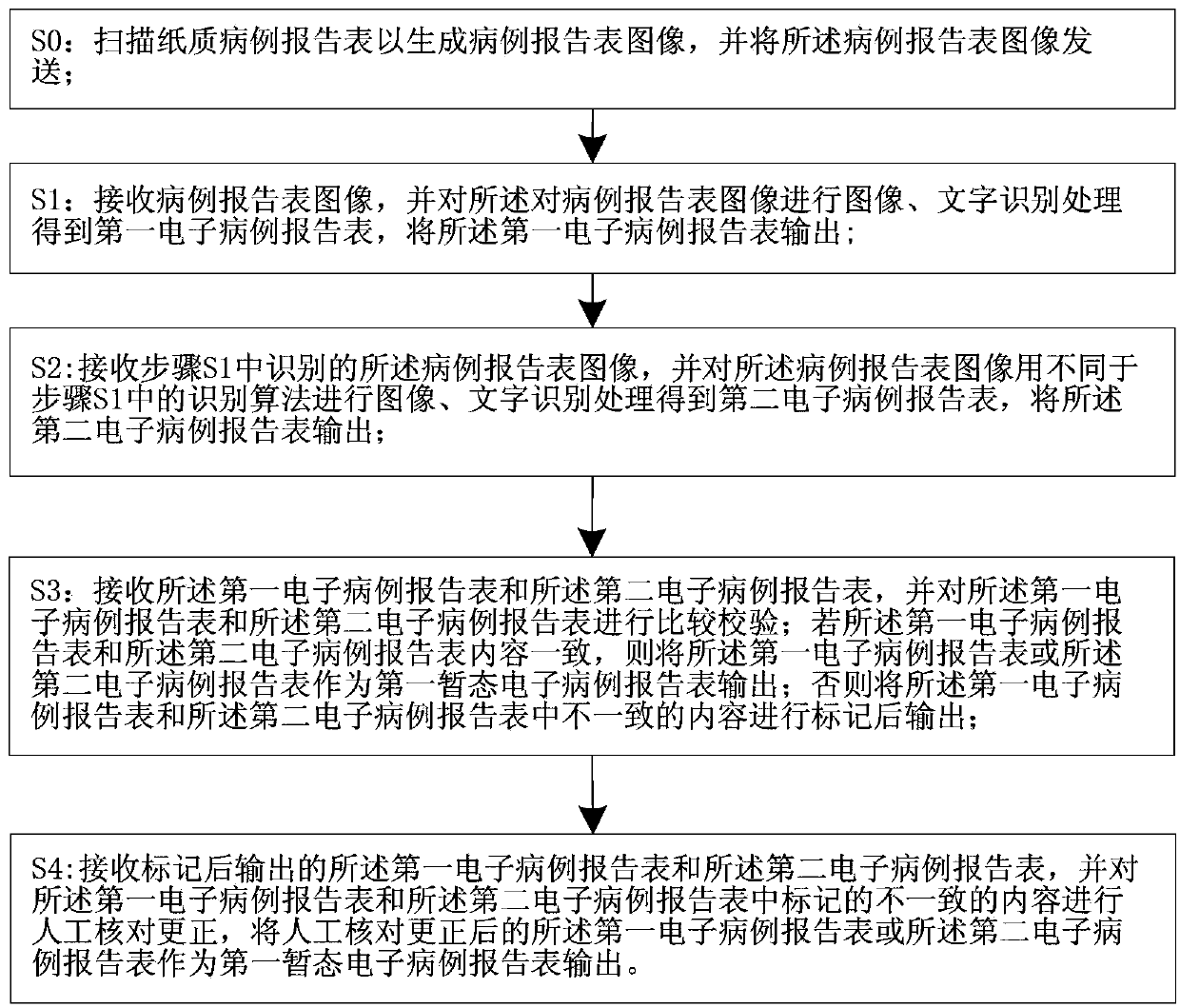
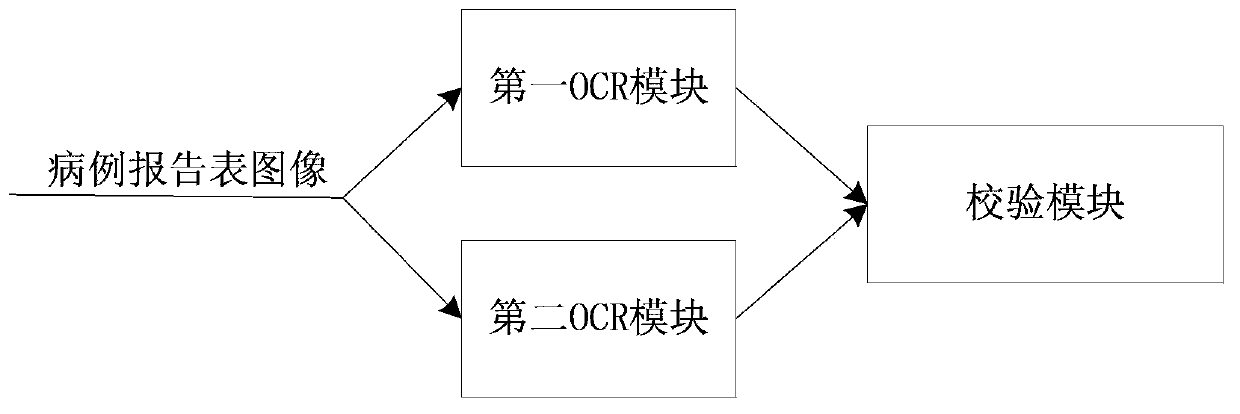
System and method for acquiring clinical case data
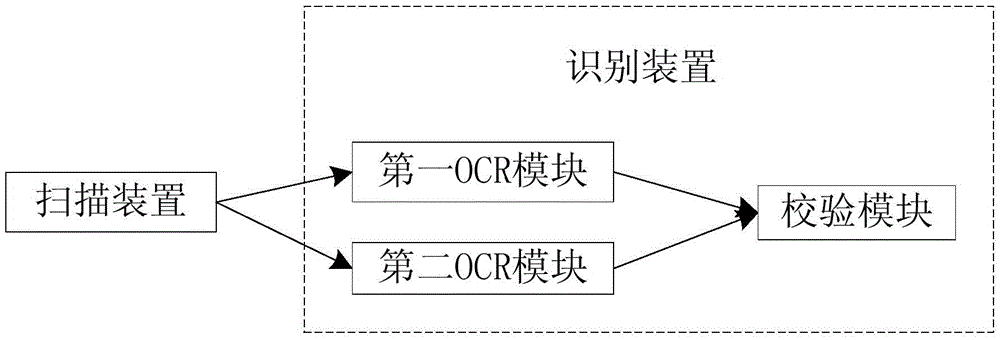
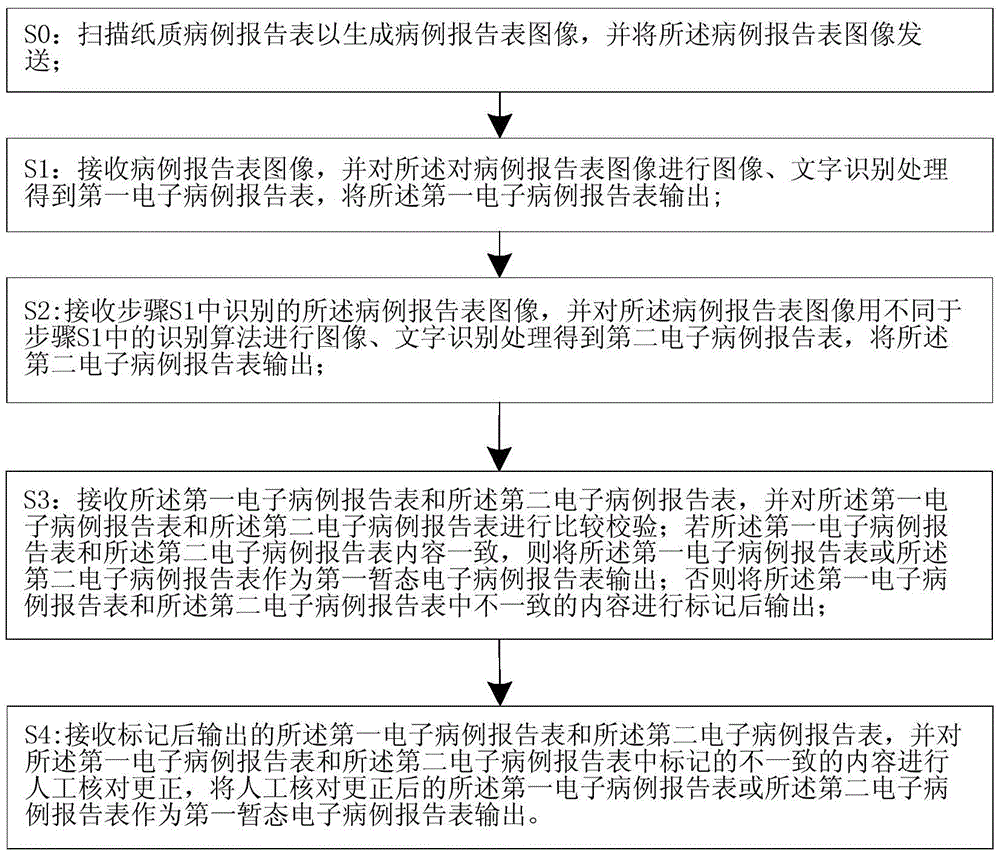
ActiveCN103425975ABig amount of dataThe recognition effect is accurateData processing applicationsCharacter and pattern recognitionData acquisitionIdentification device
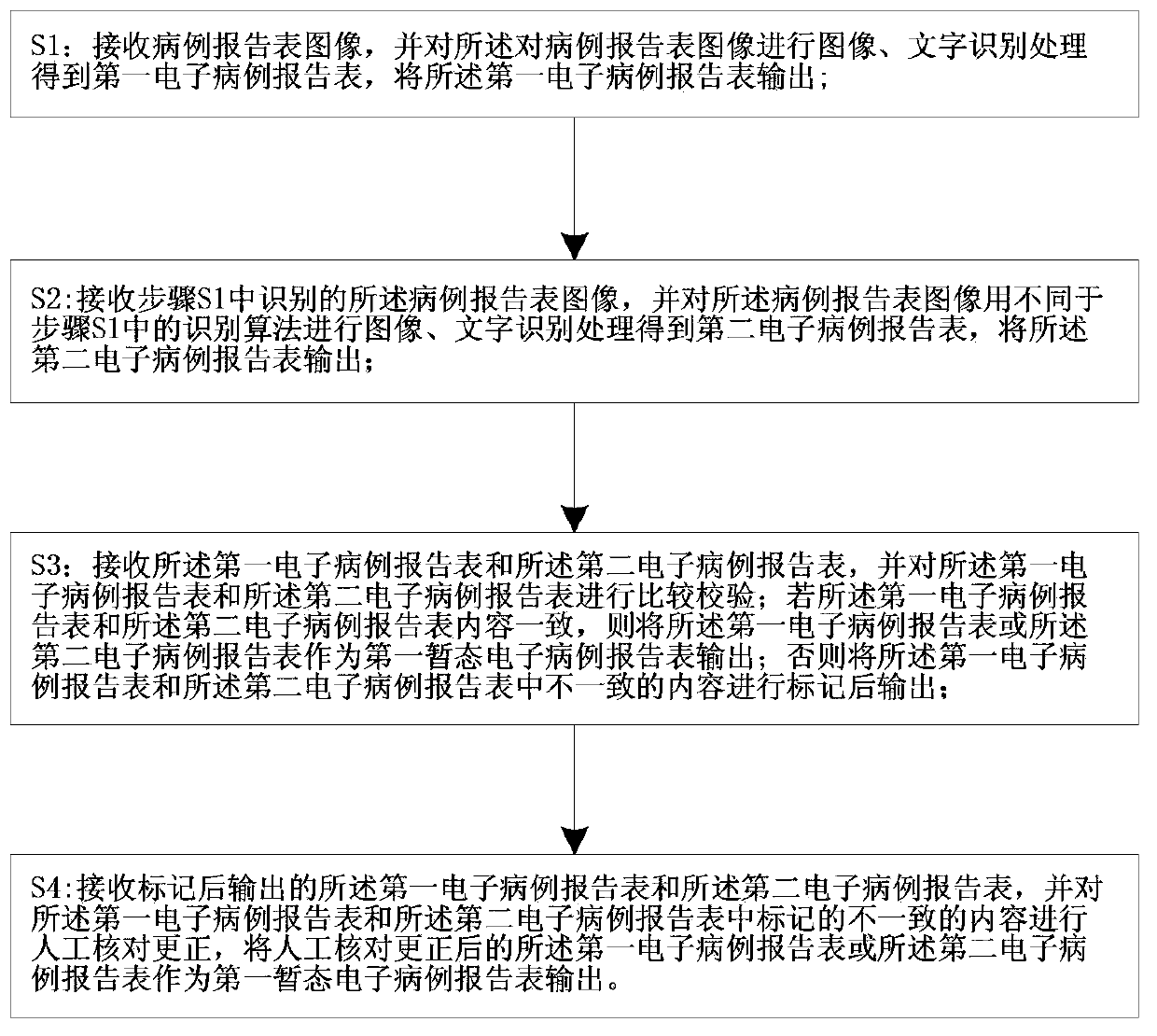
The invention relates to a system and a method for acquiring clinical case data. The system is characterized in that a verification module of a recognition device further comprises an electronic case report form comparison unit, and the electronic case report form comparison unit receives first electronic case report forms and second electronic case report forms which are transmitted by a first OCR (optical character recognition) module and a second OCR module, and compares and verifies the electronic case report forms which are transmitted by the first OCR module and the second OCR module. The system and the method have the advantages that outputted erroneous electronic case report forms can be effectively reduced while the efficiency of work for converting paper case report forms into the electronic case report forms can be improved, the accuracy of the system for acquiring the clinical case data can be improved, and the speed of the system for acquiring the clinical case data can be increased; the paper case report forms are recognized by the first OCR module and the second OCR module according to different algorithms respectively, so that the comparison accuracy of the electronic case report form comparison unit for comparing the first electronic case report forms to the second electronic case report forms can be improved.
Owner:CHINESE ACAD OF PREVENTIVE MEDICINE
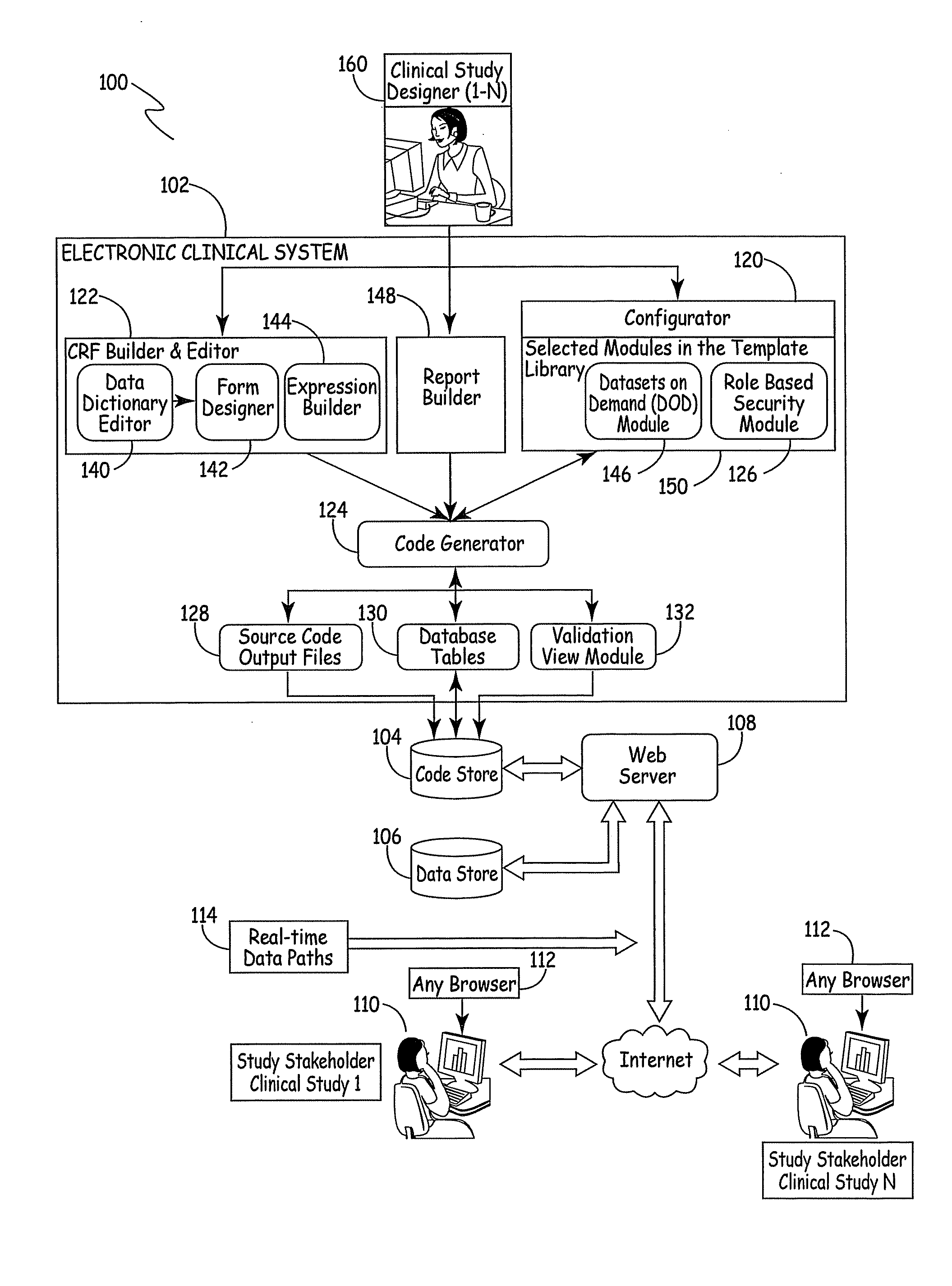
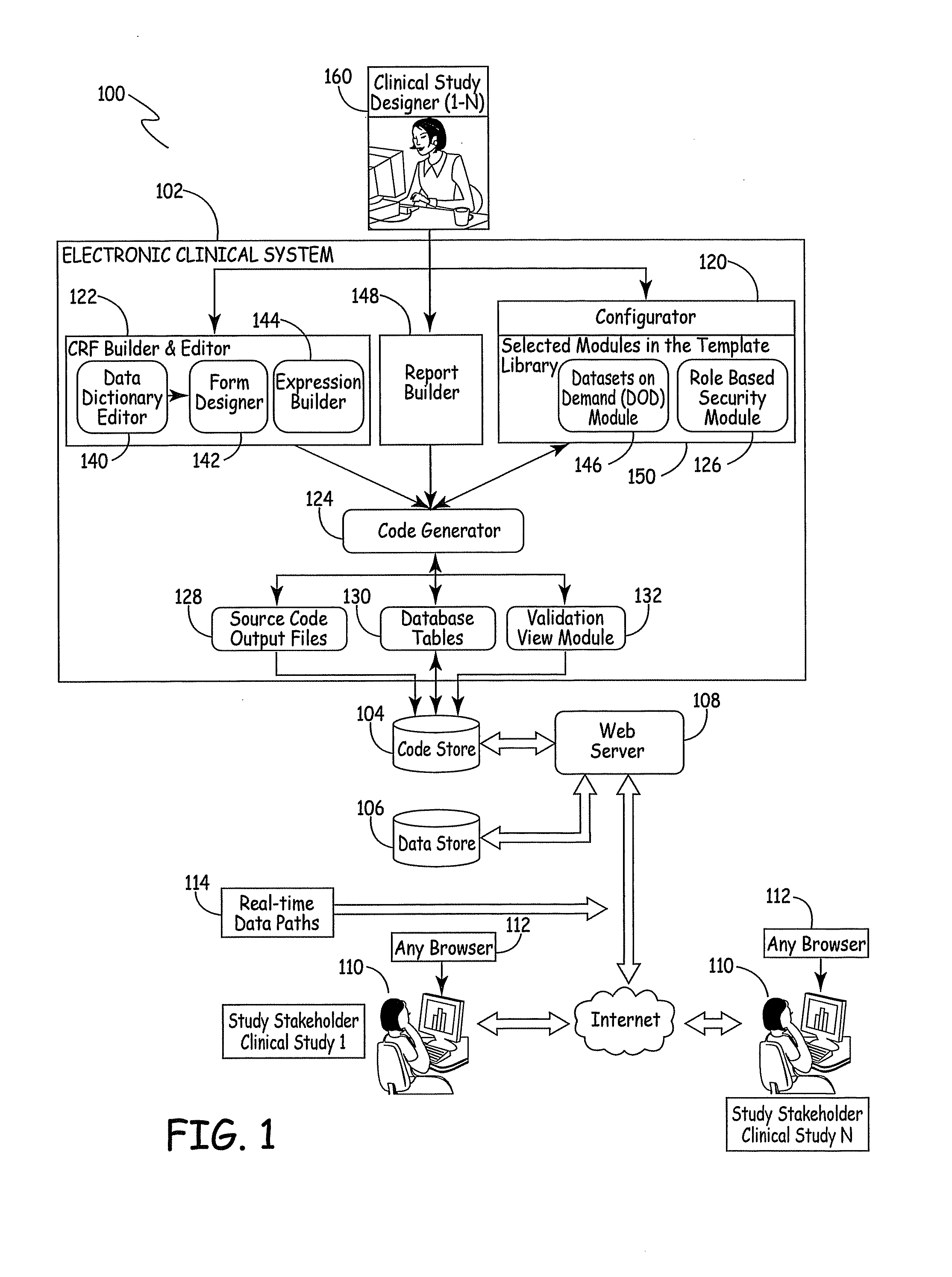
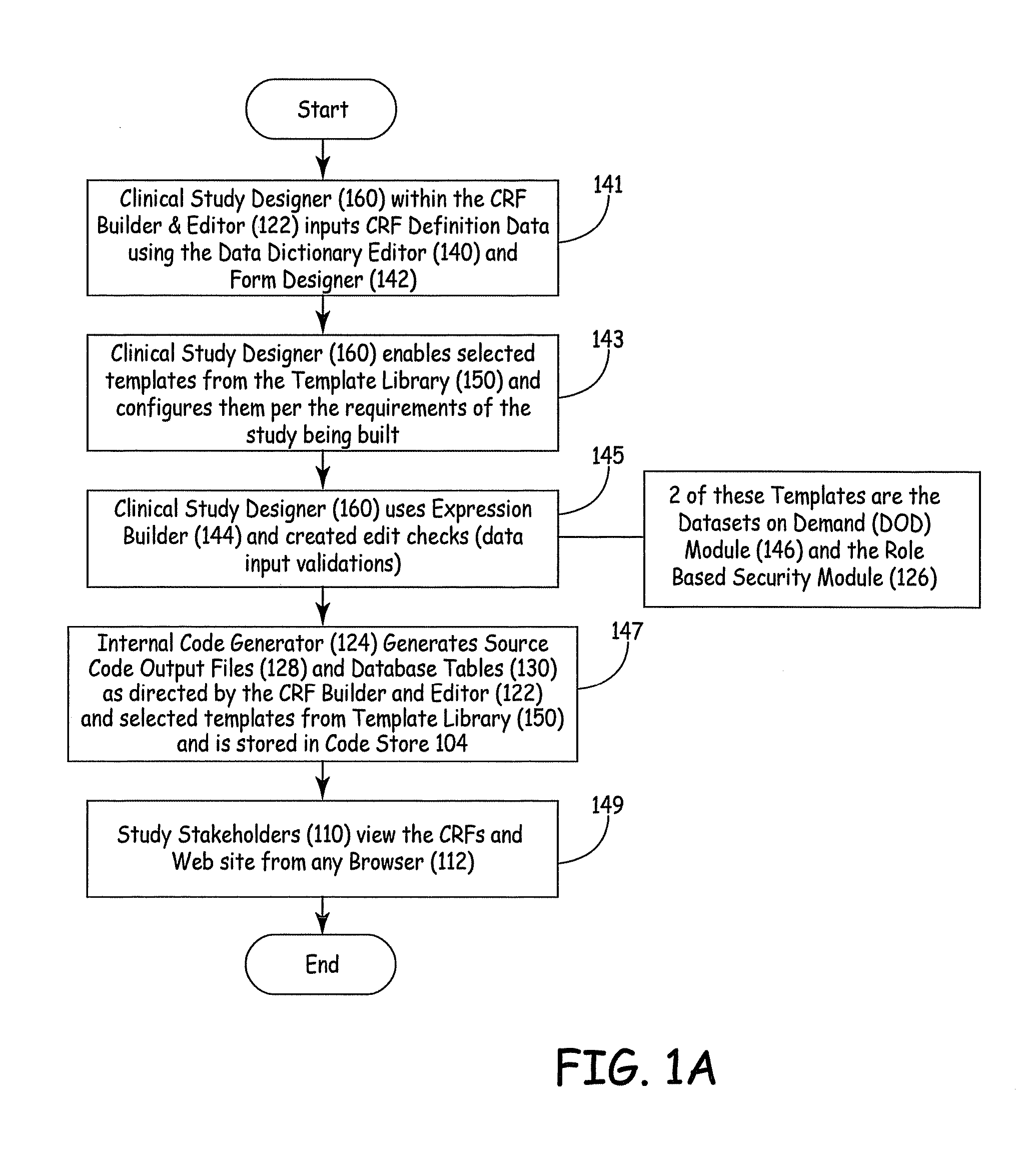
Electronic Clinical Study Site Generation System
InactiveUS20090083703A1OptimizationWell representedComputer-assisted medical data acquisitionOffice automationWeb siteClinical study
An electronic clinical system receives protocol information from a clinical study or trial designer and automatically generates source code modules and a data model for a website used in conducting the study or trial. The source code modules are used in automatically generating and exposing case report forms for use by the clinical sites participating in the study.
Owner:MEDNET SOLUTIONS INC
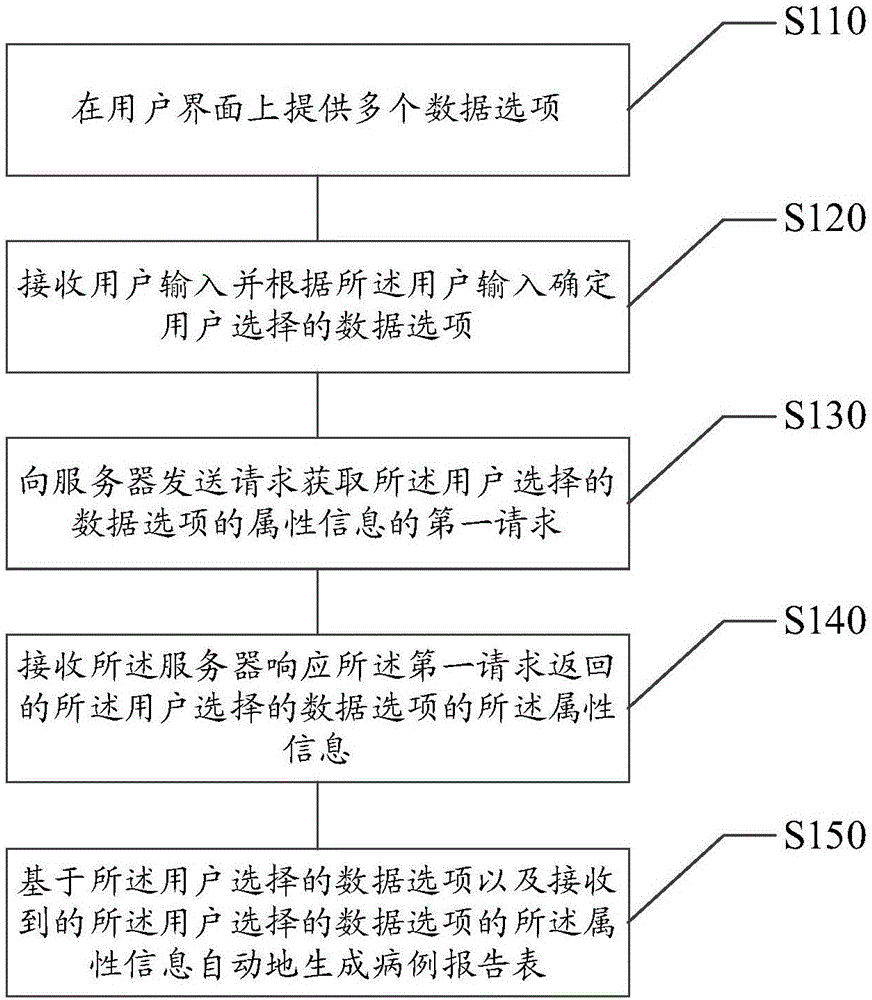
Method and device for designing case report form
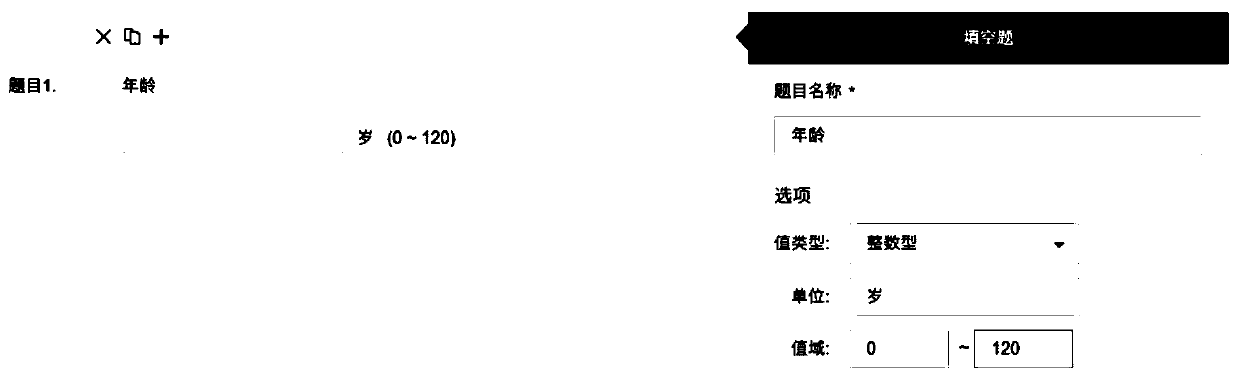
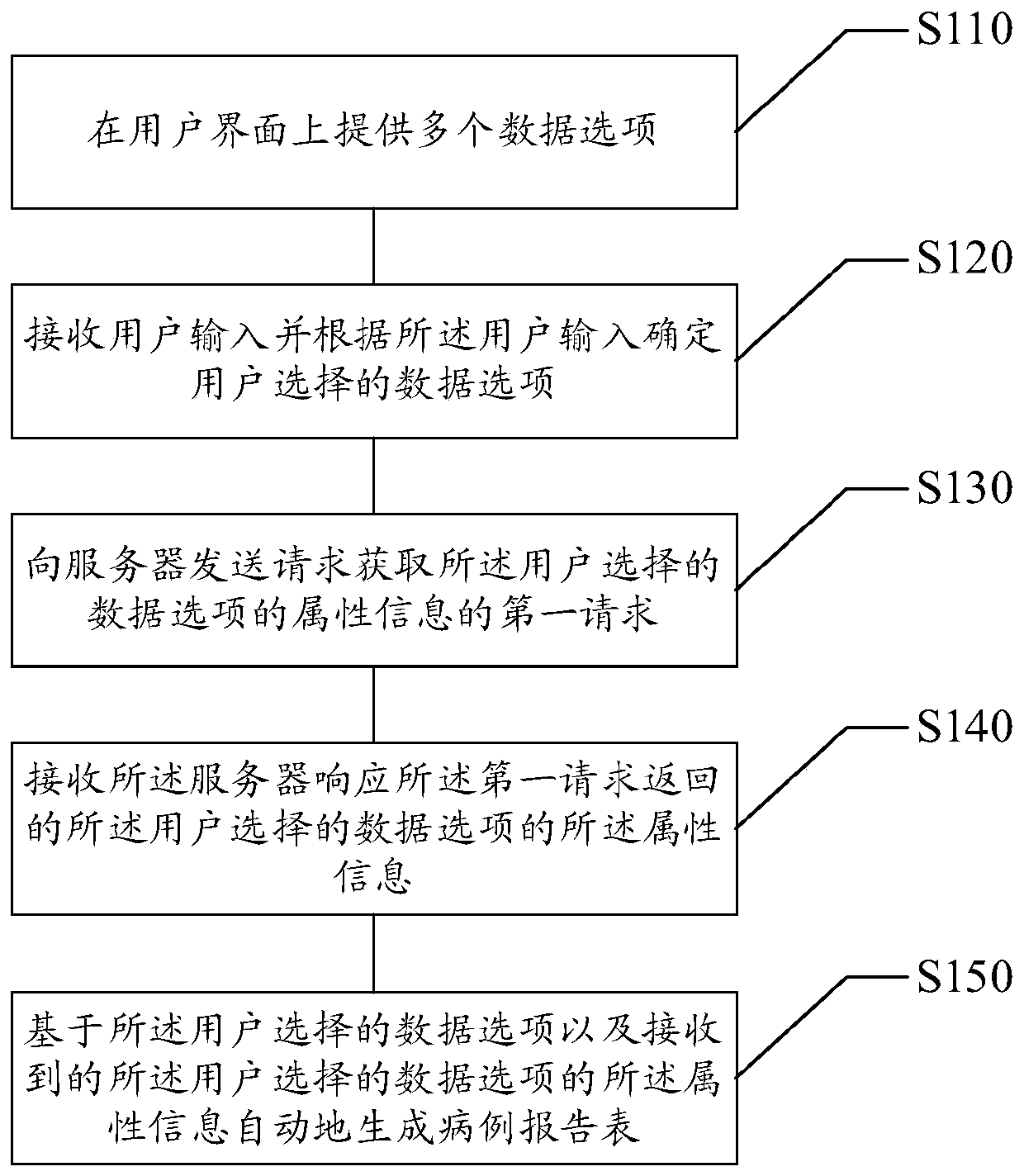
ActiveCN106445901AIn line with the habit of thinkingEasy to integrateText processingSpecial data processing applicationsUser inputTime cost
The disclosure relates to a method and a device for designing a case report form. The method comprises the following steps: providing multiple data options on a user interface; receiving a user input and determining the data options selected by a user according to the user input; sending a first request for acquiring attribute information of the data options selected by the user to a server, wherein the server stores attribute information of each data option; receiving the attribute information of the data options selected by the user, returned by the server in response to the first request; and automatically generating the case report form on the basis of the data options selected by the user and the received attribute information of the data options selected by the user. The method and the device provided by the disclosure can reduce the time cost and the manpower cost for designing the case report form, and are convenient for integration and statistical analysis of acquired data.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
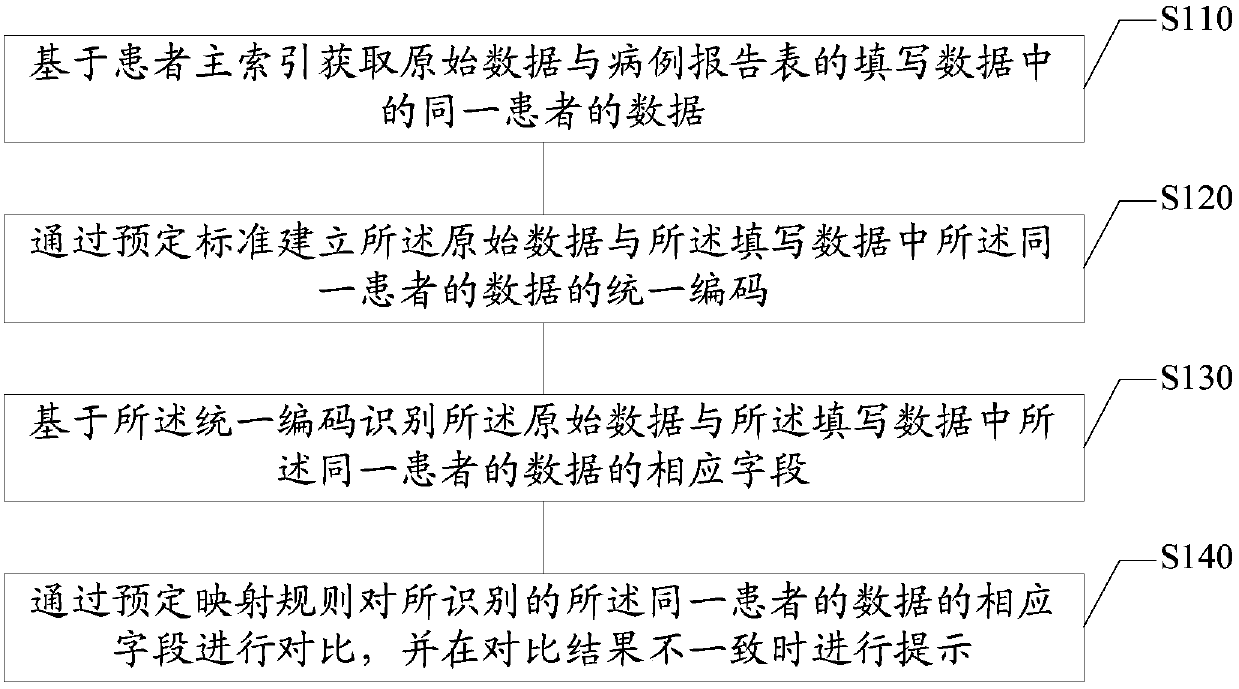
Original data checking method and device, electronic equipment and storage medium
InactiveCN107767924AReduce verification costsImprove verification efficiencyNatural language data processingSpecial data processing applicationsOriginal dataData mining
The embodiment of the invention provides an original data checking method and device, electronic equipment and a storage medium, and relates to the technical field of data processing. The method comprises the steps that the data of the same patient in the original data and the fill-in data of a case report form are acquired based on the master patient index; the unified code of the data of the same patient in the original data and the fill-in data is established through the preset standard; the corresponding fields of the data of the same patient in the original data and the fill-in data are identified based on the unified code; and the identified corresponding fields of the data of the same patient are compared through the preset mapping rules and prompting is performed when the comparison result is inconsistent. According to the technical scheme, SDV checking can be automatically performed so that the SDV checking cost can be reduced and the SDV checking efficiency can be enhanced.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
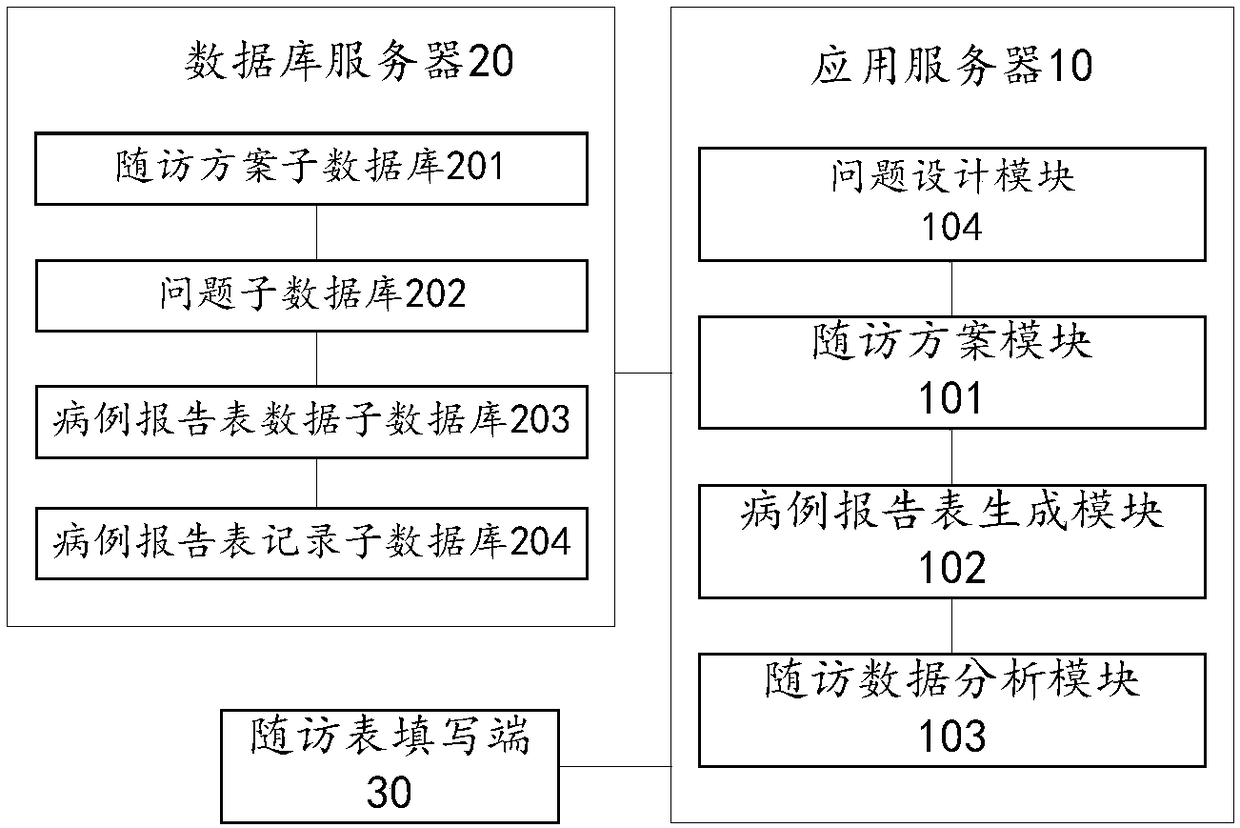
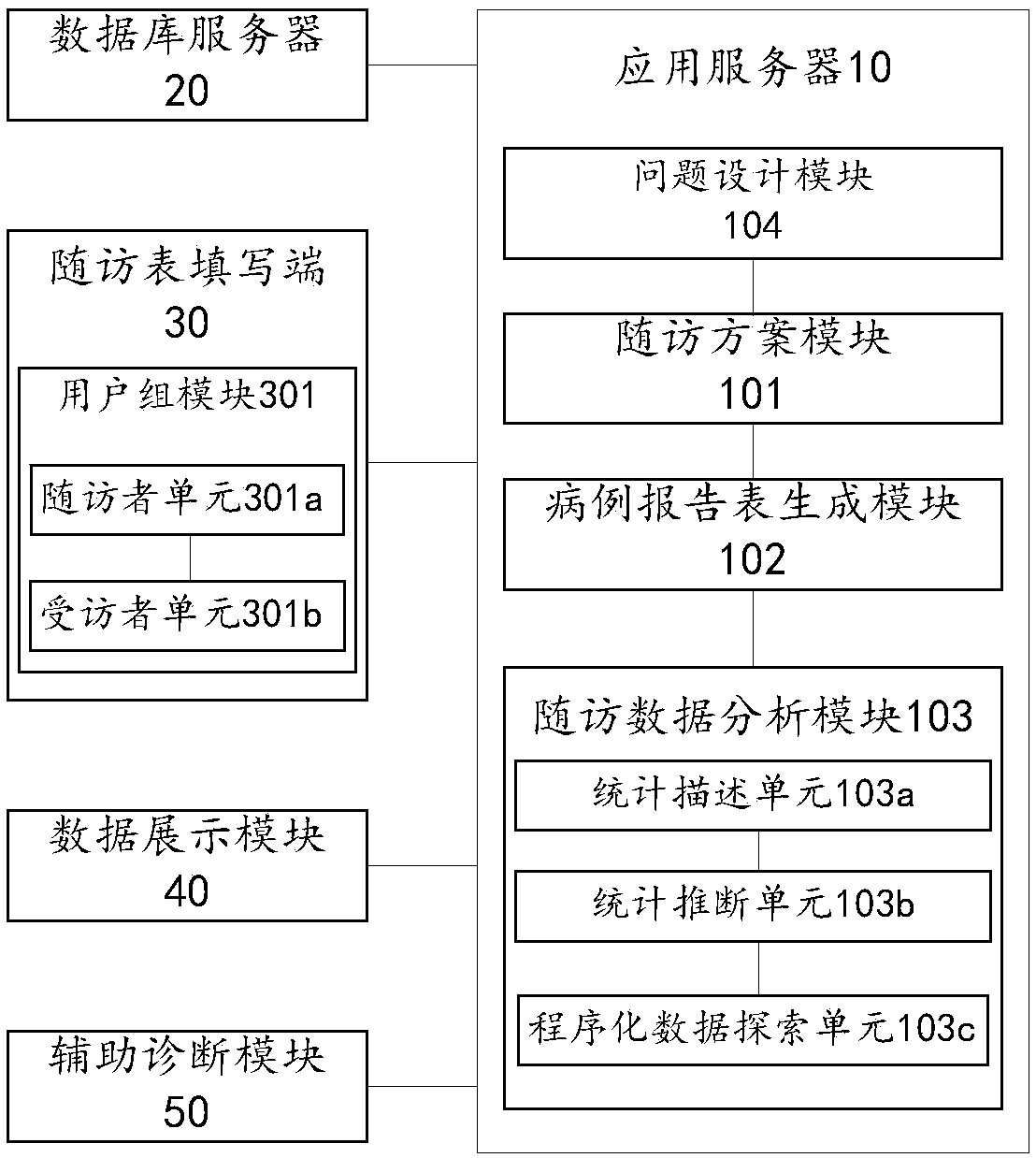
Electronic medical record report form system and electronic medical record report form recording method
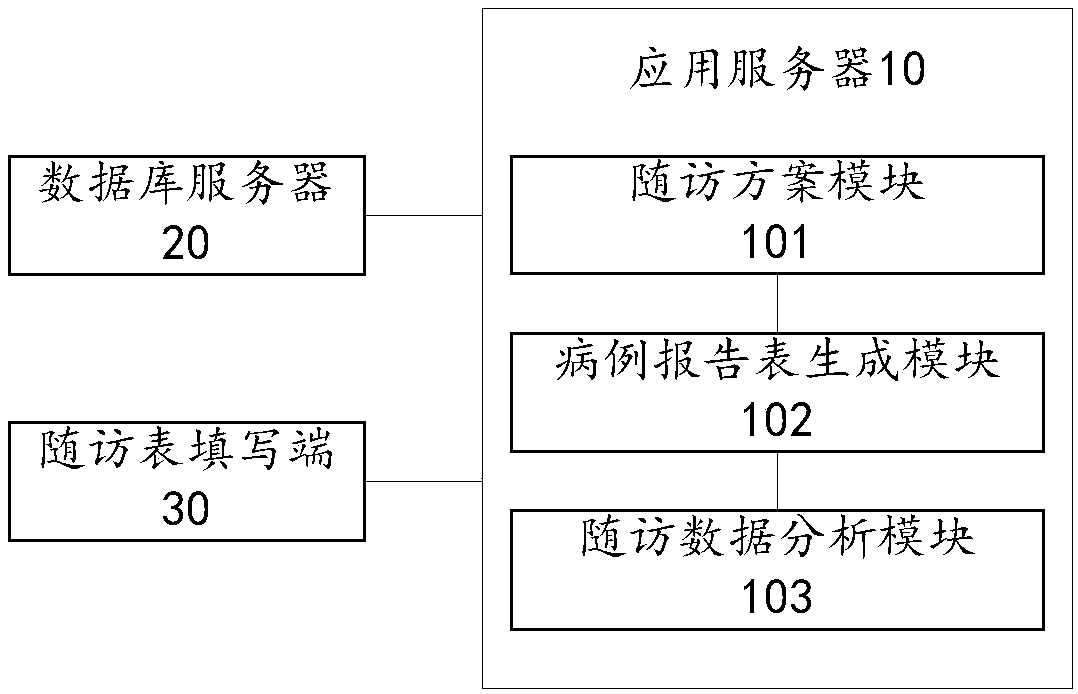
InactiveCN108154906AImprove universal applicabilityEasy to carryInstrumentsMedical reportsMedical recordApplication server
The invention provides an electronic medical record report form system and an electronic medical record report form recording method and relates to the technical field of medical communication, the system comprises an application server, a database server and a follow-up visit form filling end, wherein the database server and the follow-up visit form filling end are in communication connection with the application server; the database server is used for storing predesigned follow-up visit schemes and filled electronic medical record report forms; the follow-up visit form filling end is used for calling the follow-up visit scheme matched with a follow-up visit instruction according to the follow-up visit instruction input by a follow-up visitor, receiving the medical record data filled by the follow-up visitor according a medical record report form template and transmitting the medical record data to the application server; the application server comprises a follow-up visit scheme template, a medical record report form generation module and a follow-up visit data analysis module. According to the electronic medical record report form system and the electronic medical record report form recording method, the values of clinical follow-up visit data can be excavated favorably, and the general applicability of the electronic medical record report form system and the clinical follow-up visit efficiency are improved.
Owner:林沛杰
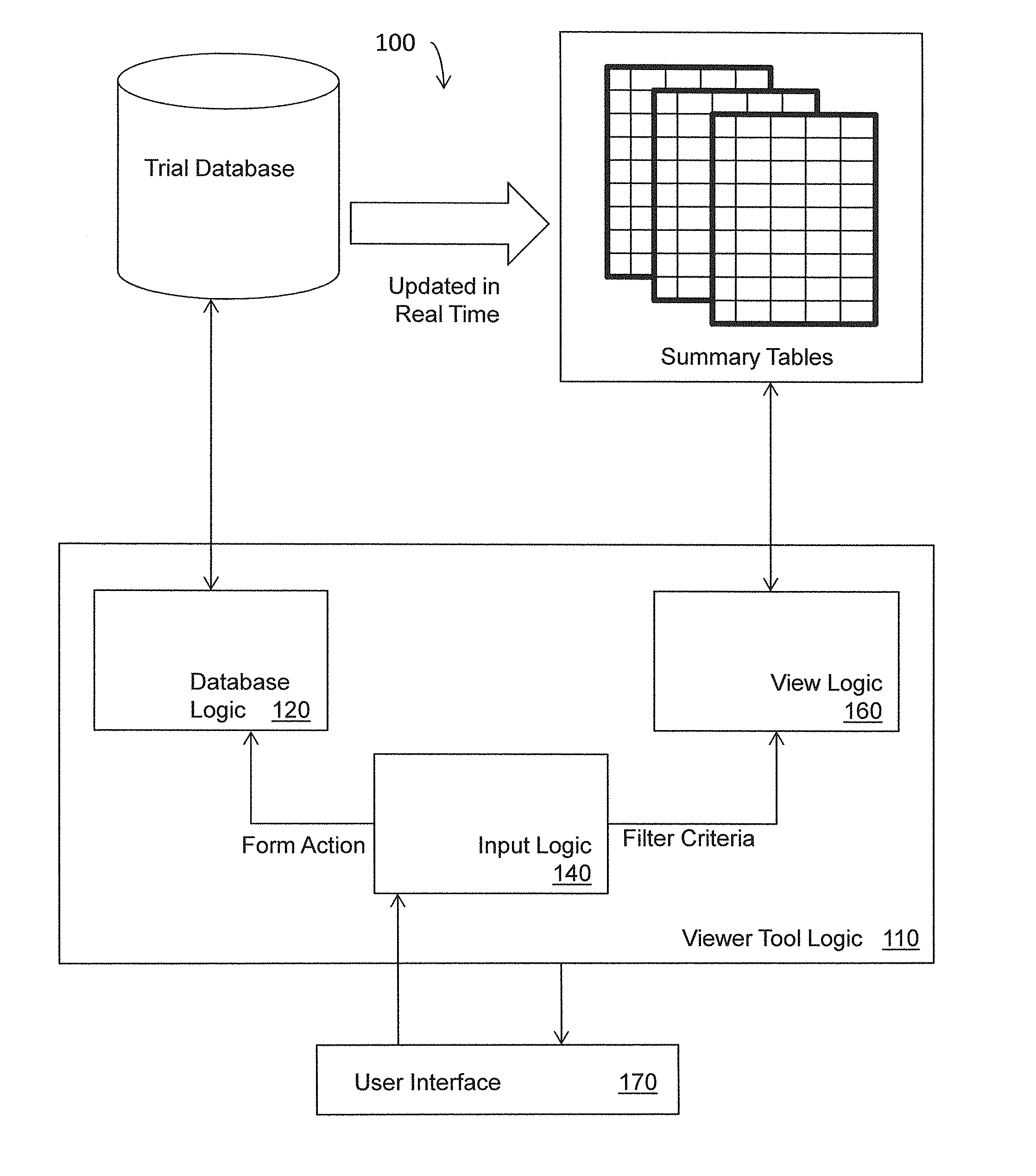
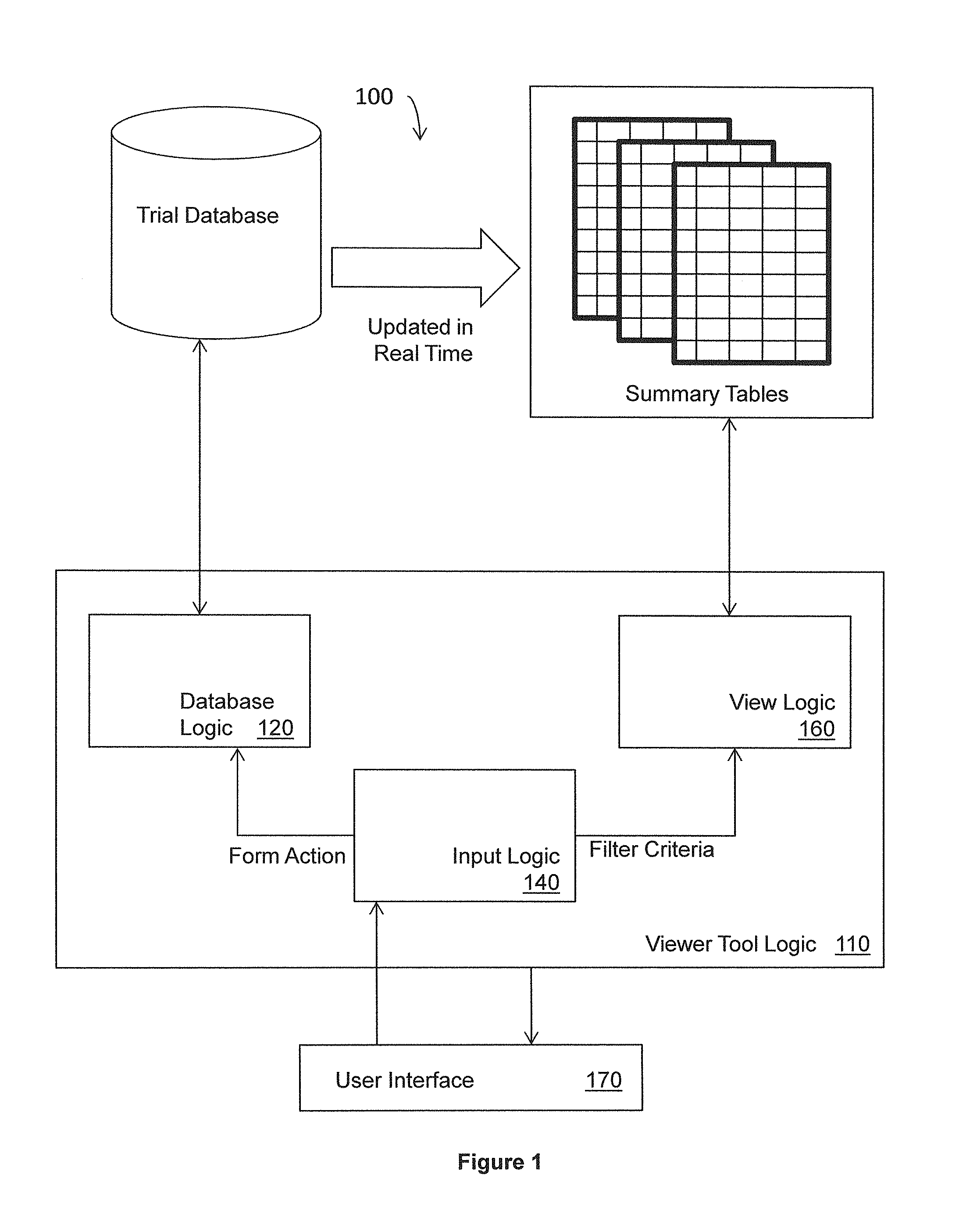
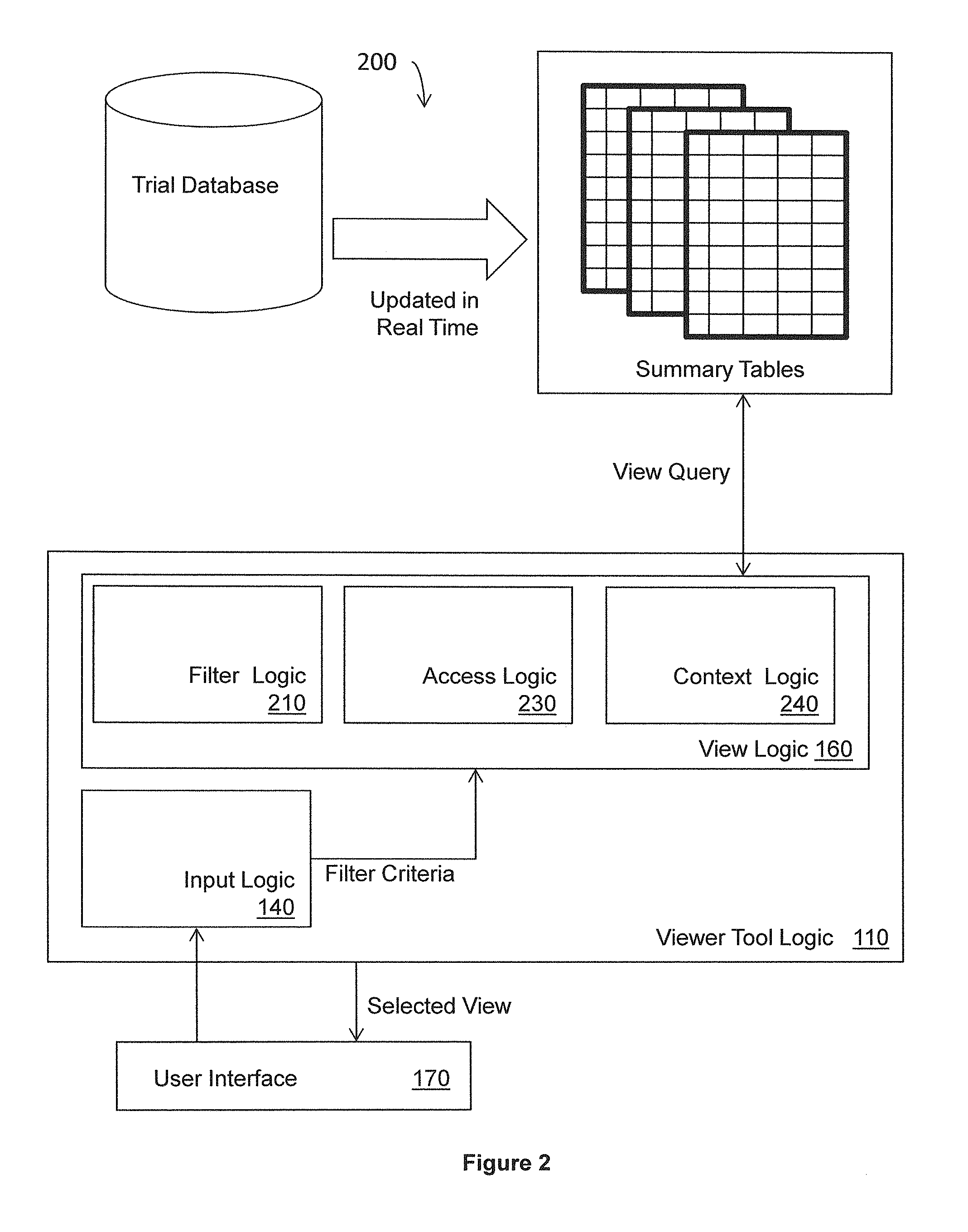
Data viewer for clinical data
ActiveUS20130318106A1Digital data information retrievalDigital data processing detailsData viewSummary data
Systems, methods, and other embodiments associated with a data view for clinical data are described. In one embodiment, a method includes receiving a request from a user to view summarized case report form (CRF) data, where the CRF data corresponds to a plurality case report forms (CRFs) stored in one or more normalized transactional tables. A view query is constructed based, at least in part, on the request and executed on one or more denormalized summary tables to construct a view. The denormalized summary tables store selected CRF data from the normalized transactional tables and are updated concurrently with each update to the transactional tables. The method includes rendering the view for display.
Owner:ORACLE INT CORP
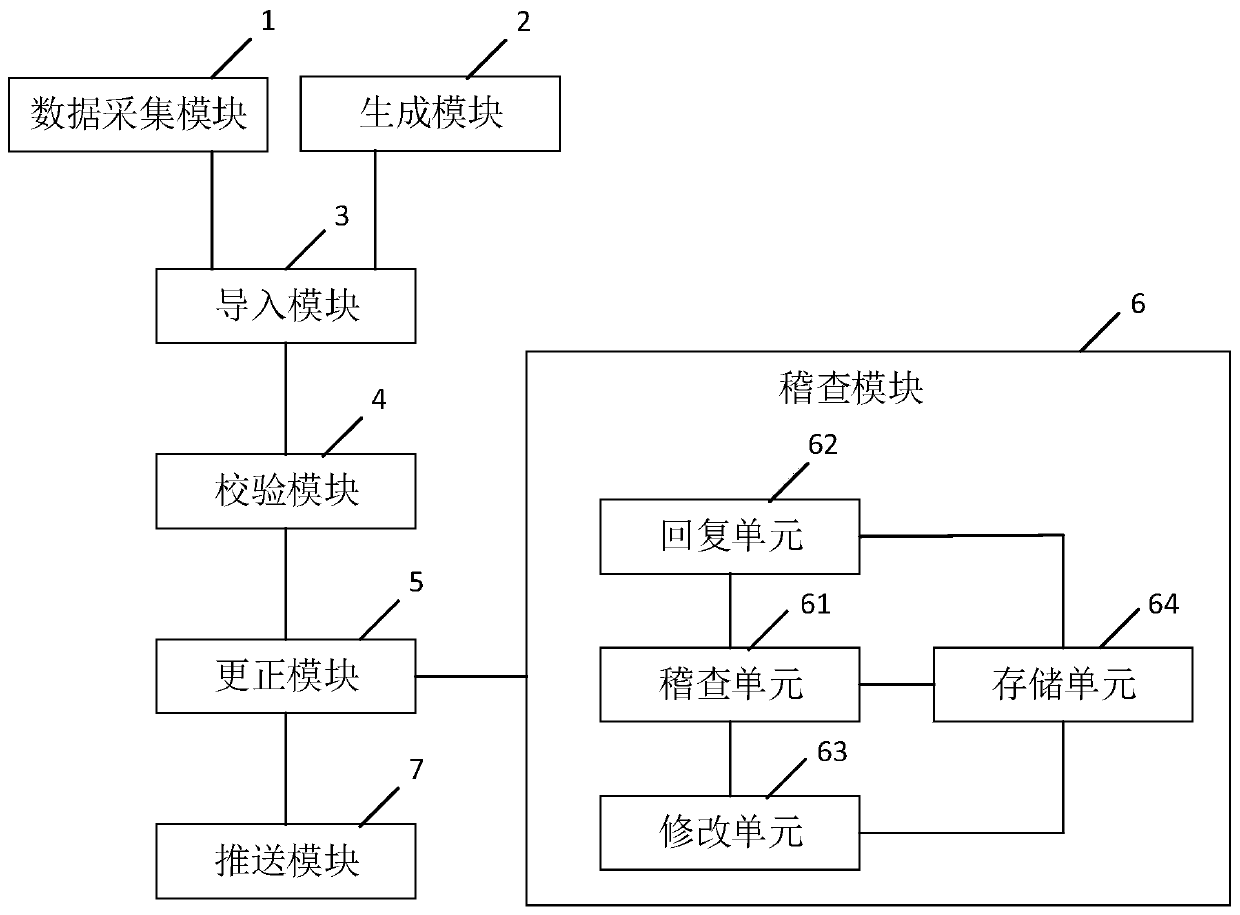
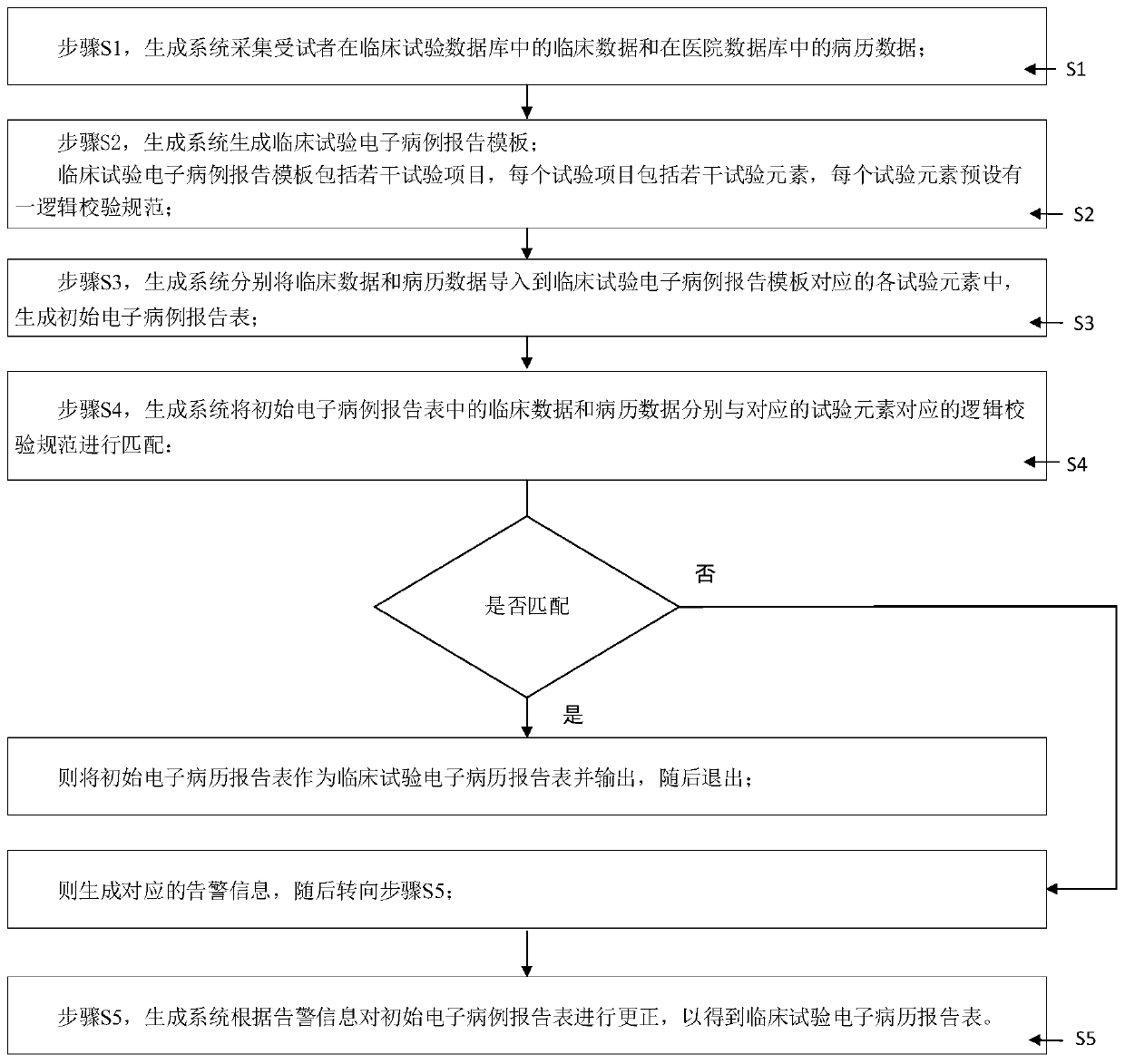
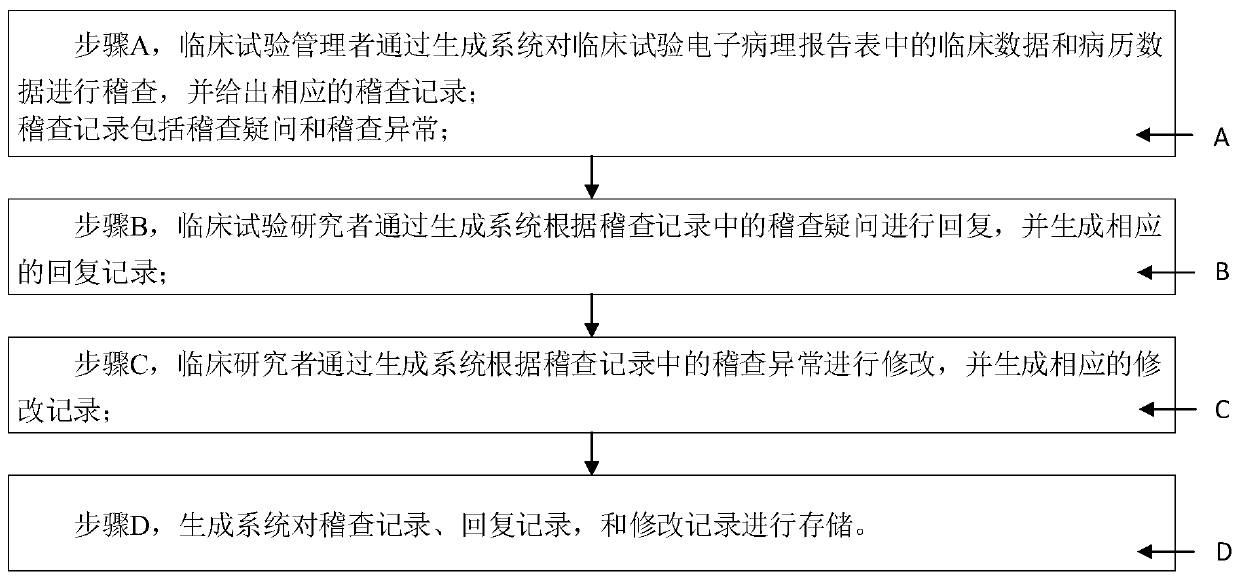
System and method for generating clinical test electronic case report form
ActiveCN110826309AAchieve securityAchieve reliabilityNatural language data processingText database indexingMedical recordClinical tests
The invention discloses a system and a method for generating a clinical test electronic case report form, and relates to an electronic case report form. A data acquisition module is used for acquiringclinical data and medical record data of a subject; a generation module is used for generating a clinical test electronic case report template which comprises a plurality of test items, each test item comprises a plurality of test elements, and a logic verification specification is preset for each test element; the import module is used for importing the clinical data and the medical record datainto each test element to generate an initial electronic medical record report form; the verification module is used for respectively matching the clinical data and the medical record data with logicverification specifications corresponding to the corresponding test elements, and generating and outputting alarm information when the clinical data and the medical record data do not accord with thelogic verification specifications; and the correction module is used for performing correction according to the alarm information to obtain a clinical test electronic medical record report table. Thesystem and the method have the following beneficial effects: automatic acquisition and inspection management of all data in the clinical test electronic case report form are realized.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL +1
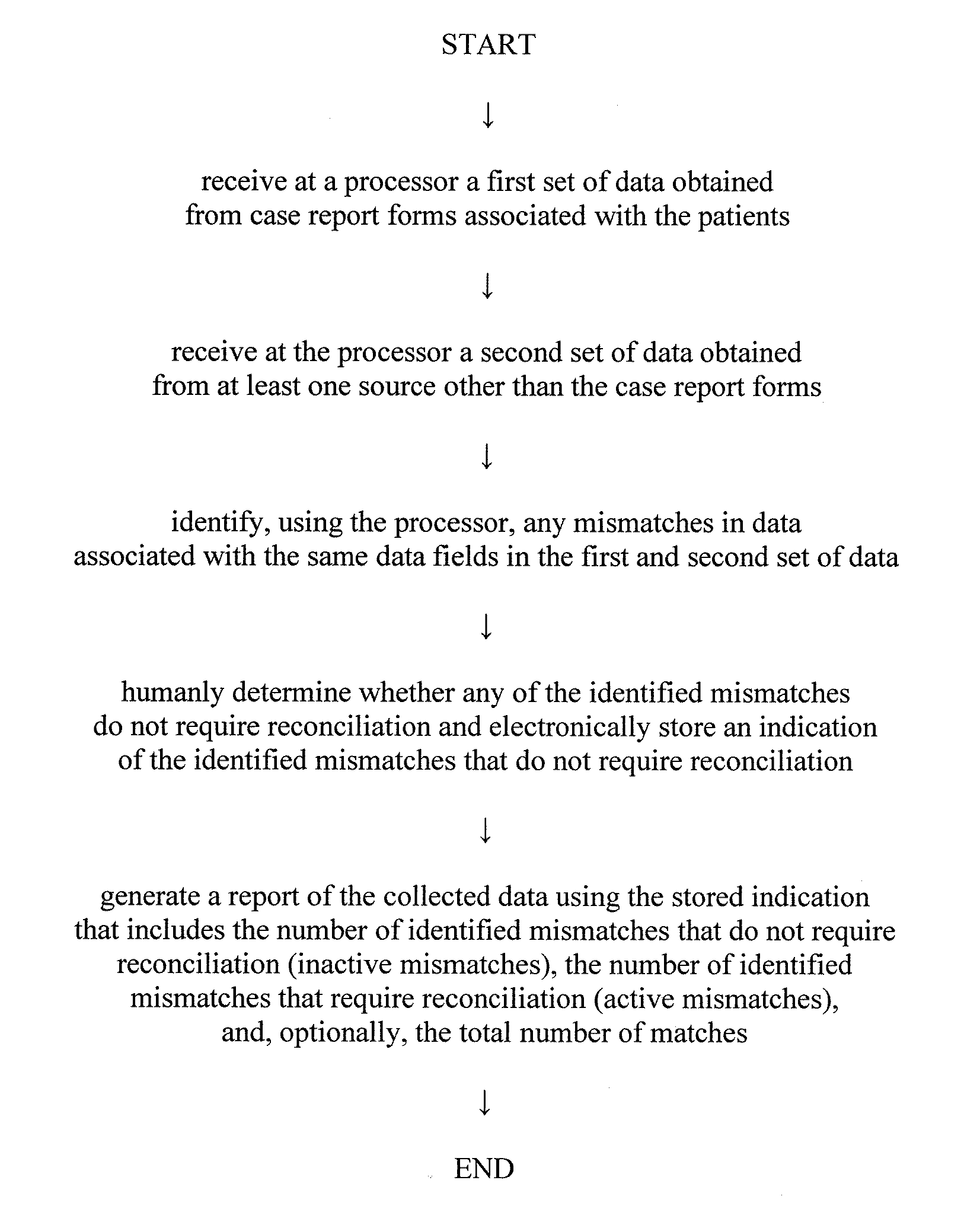
Automated method of generating reconciliation reports regarding mismatches of clinical data received from multiple sources during a clinical trial
ActiveUS20120246149A1Digital data processing detailsComputer-assisted medical data acquisitionClinical trialData field
An automated method is provided of generating a report of data collected from multiple sources during a clinical trial. The data includes a plurality of different data fields. A plurality of patients participate in the clinical trial. A first set of data obtained from case report forms associated with the patients is received at a processor. The case report forms are one source of data. A second set of data obtained from at least one source other than the case report forms is also received at the processor. At least some of the data in the first and second set of data include data associated with the same data fields. The processor identifies any mismatches in data associated with the same data fields in the first and second set of data. Mismatches associated with data fields obtained from the same case report forms are electronically grouped. A report is then generated of the data. The report shows the status of mismatches for each case report form or type of case report form.
Owner:NUMODA TECH
Visit schedule generation method and system and computer readable medium
PendingCN111079389AIn line with reading habitsReduce learning costsText processingVisit timeBusiness Personnel
The present invention relates to a visit schedule generation method, the visit schedule corresponding to a clinical trial research process, the method comprising: providing a visit plan project creation page, and receiving a first input of a project name at the visit plan project creation page; providing a visit stage configuration page, and receiving a second input for the visit times in the visit stage configuration page; providing a case report form configuration page, and receiving a third input of an inspection item of the case report form on the case report form configuration page; and generating a visit schedule, wherein the visit schedule presents the second input and the third input at the same time according to the corresponding relationship. According to the method provided by the invention, the visit schedule can be conveniently and quickly configured in batches, the generated visit schedule conforms to the reading habit of the business personnel, and the learning cost of the business personnel is saved.
Owner:上海太美星云数字科技有限公司
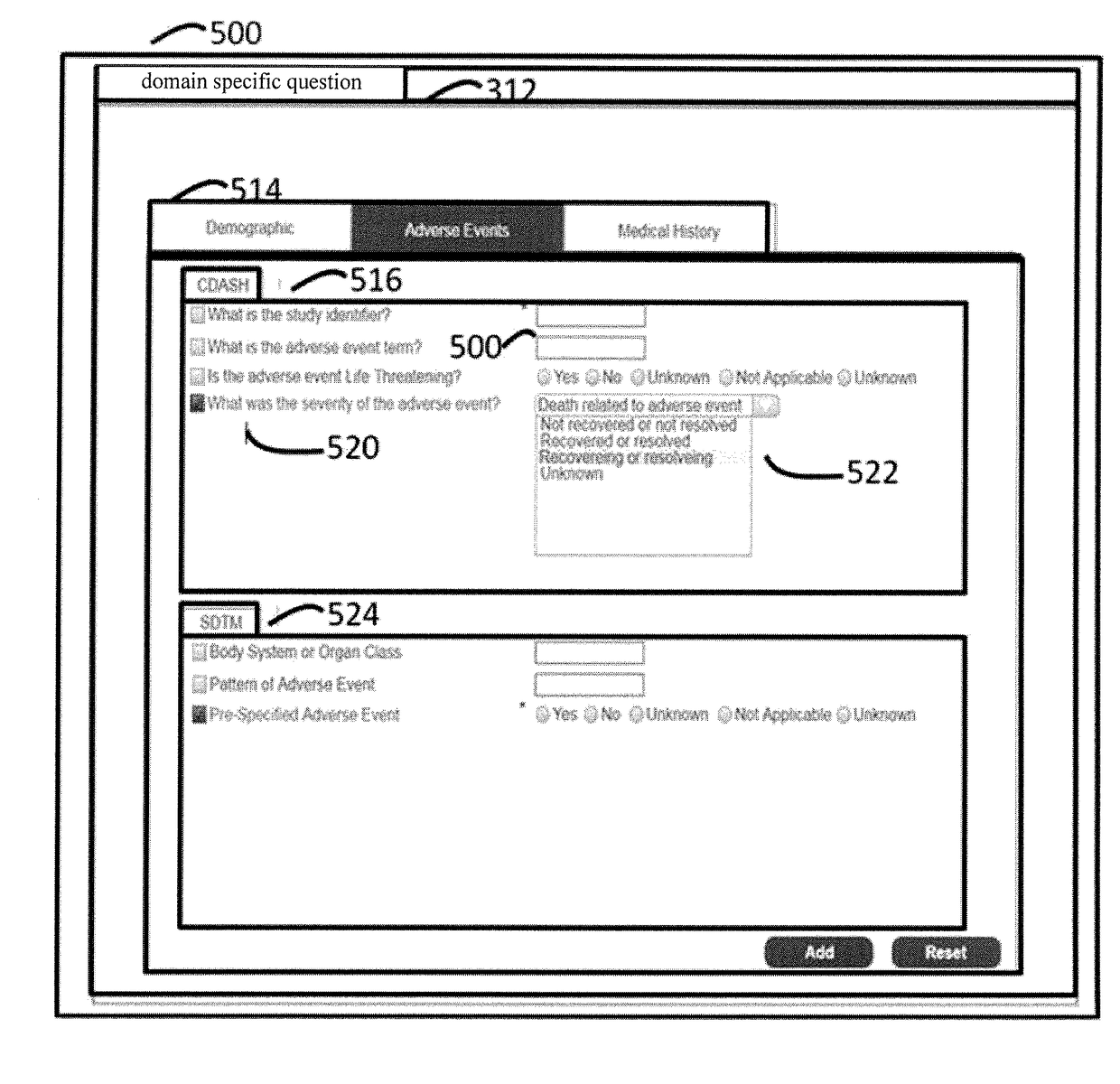
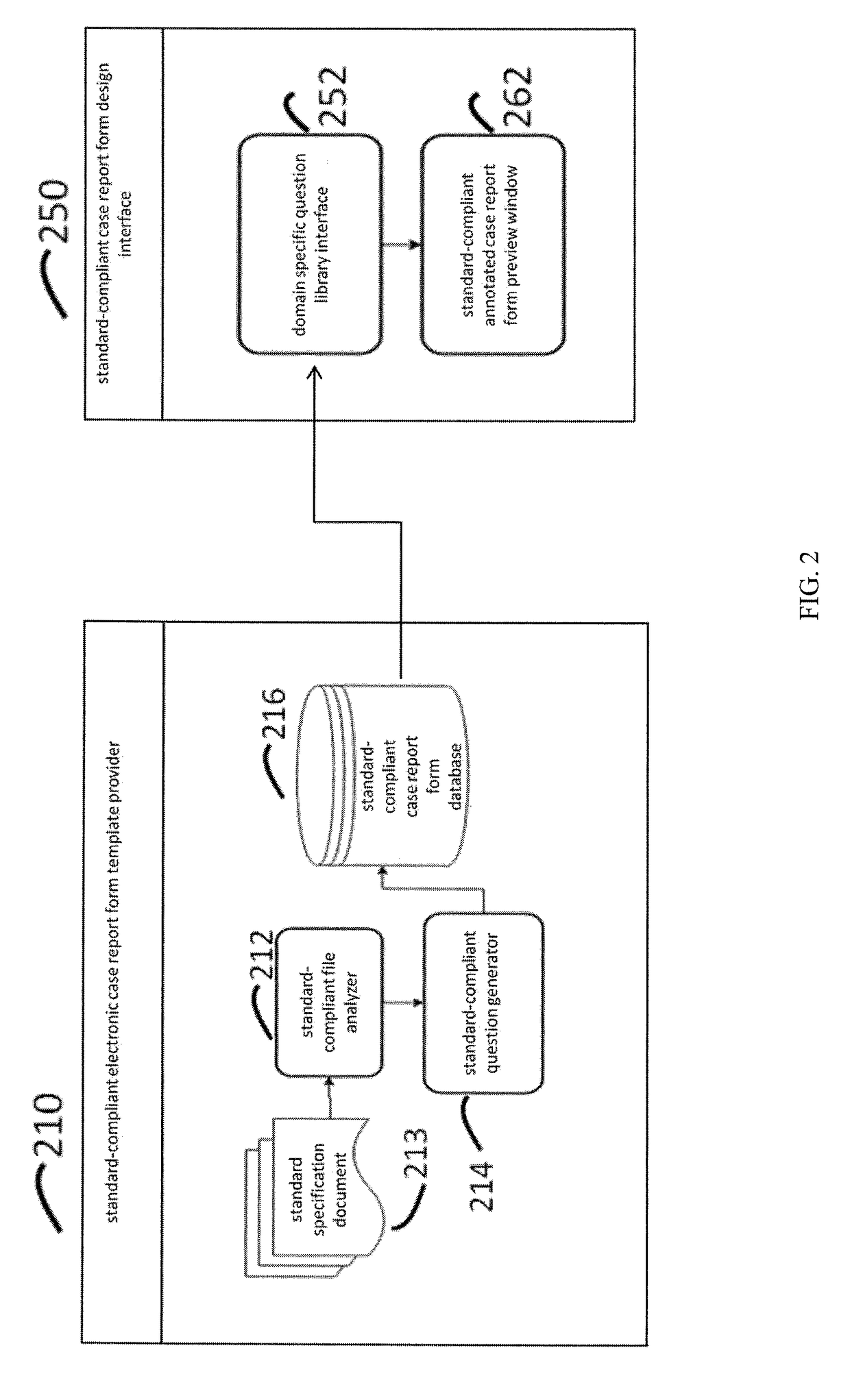
System and method of standard-compliant electronic case report form design and clinical data set generation
InactiveUS20180060539A1Computer-assisted medical data acquisitionMedical report generationData setSpecification document
In a method of standard-compliant electronic case report form (eCRF) design and clinical data set collection, the steps comprise providing a standard specification document. Then, analyzing the standard specification document to generate a standard-compliant question information by a standard specification analyzer, and transforming the standard-compliant question information to a standard-compliant question object by a standard-compliant question generator. Generating a standard-compliant eCRF by a standard-compliant case report form design interface. Moreover, confirming the standard-compliant eCRFs whether comply with the standard specification by a standard rules validator.
Owner:NATIONAL YANG MING UNIVERSITY
System and method for recognizing clinical case report forms
ActiveCN103425976ABig amount of dataThe recognition effect is accurateCharacter and pattern recognitionSpecial data processing applicationsComputer moduleLibrary science
The invention relates to a system and a method for recognizing clinical case report forms. The system is characterized in that a verification module comprises an electronic case report form comparison unit, and the electronic case report form comparison unit receives first electronic case report forms and second electronic case report forms which are transmitted by a first OCR (optical character recognition) module and a second OCR module, and compares and verifies the electronic case report forms which are transmitted by the first OCR module and the second OCR module. The system and the method have the advantages that outputted erroneous electronic case report forms can be effectively reduced while the efficiency of work for converting paper case report forms into the electronic report case forms can be improved, the accuracy of the system for recognizing the clinical case report forms can be improved, and the speed of the system for recognizing the clinical case report forms can be increased; the paper case report forms are recognized by the first OCR module and the second OCR module according to different algorithms respectively, so that the comparison accuracy of the electronic case report form comparison unit for comparing the first electronic case report forms to the second electronic case report forms can be improved.
Owner:CHINESE ACAD OF PREVENTIVE MEDICINE
Method and device for automatically generating case report form, computer-readable storage medium, and electronic device
ActiveCN110675924AEasy to storeEasy to analyzeSemantic analysisOther databases indexingSoftware engineeringResearch data
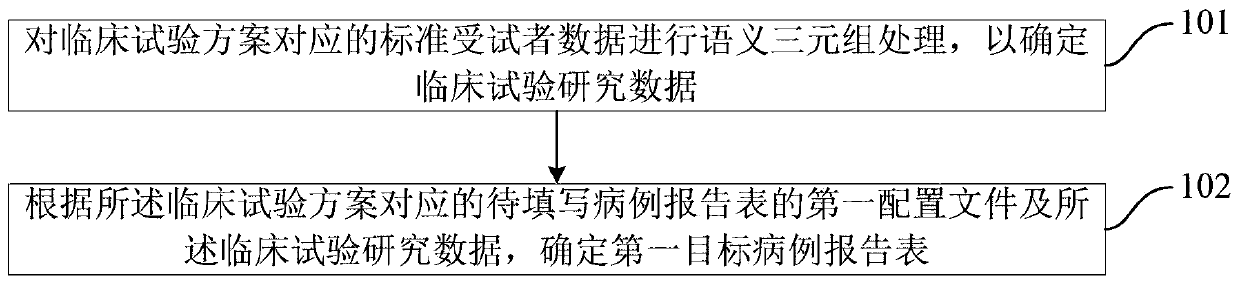
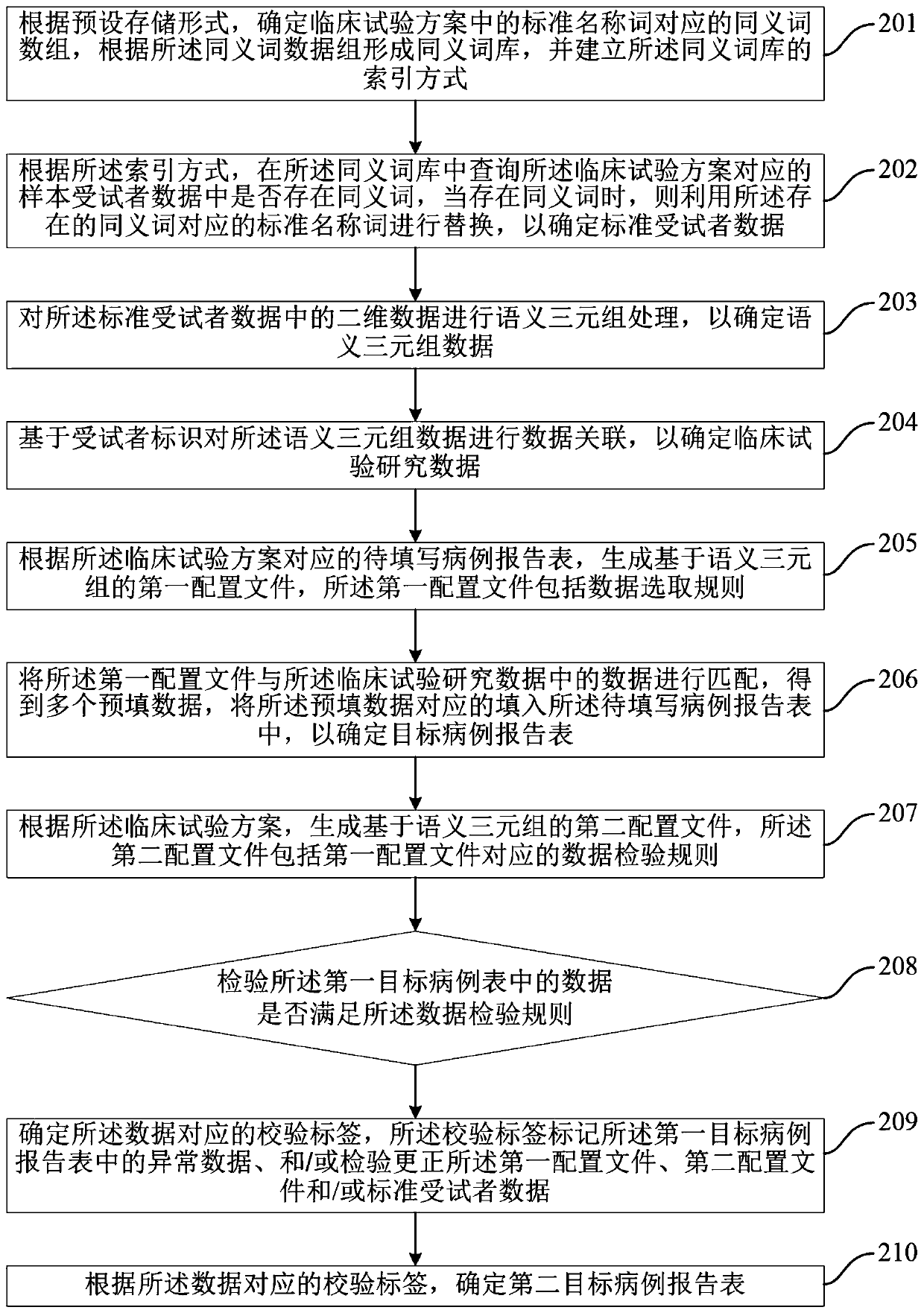
The invention discloses a method and device for automatically generating a case report form, a computer-readable storage medium, and an electronic device. The method includes: performing a semantic triple process on standard subject data corresponding to a clinical trial scheme to determine clinical trial research data; and determining a first target case report form according to a first configuration file of a to-be-filled case report form corresponding to the clinical trial scheme and the clinical trial research data. The method automatically generates the target case report form, does not need to fill in the target case report form manually, and avoids errors in a manual filling process, thereby improving the entry efficiency and data quality of the target case report form.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD +1
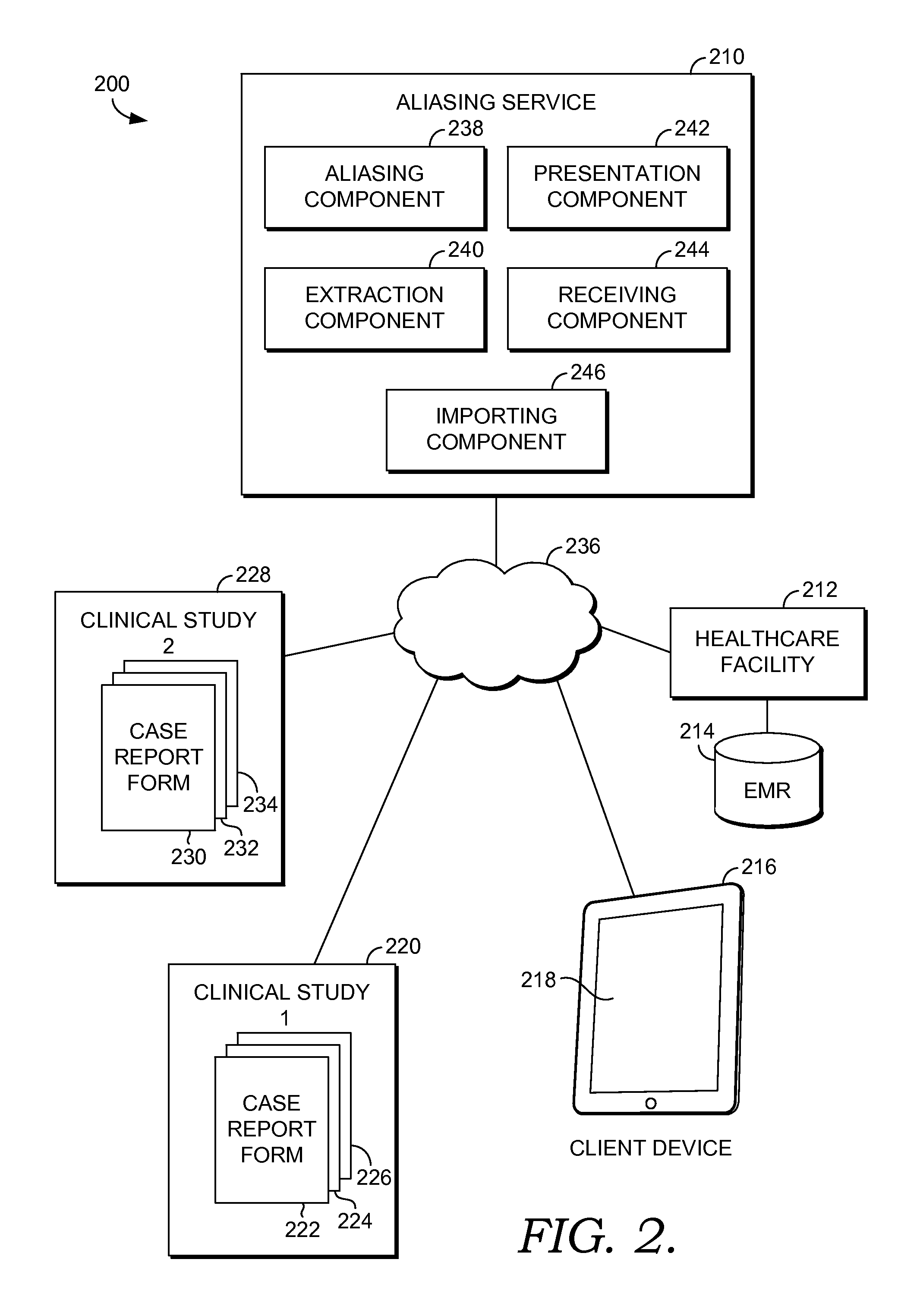
Integrated data capture using aliasing schemes
InactiveUS20150019253A1Well formedFacilitate electronic transcriptionData processing applicationsNatural language data processingMedical recordTask completion
Methods, systems, and computer-storage media are provided for using a generic aliasing scheme to facilitate electronic transcription of groups of clinical event data extracted from an electronic medical record to case report forms associated with clinical studies. The generic aliasing scheme is also used to electronically transcribe documentation of task completion to case report forms associated with the clinical studies.
Owner:CERNER INNOVATION
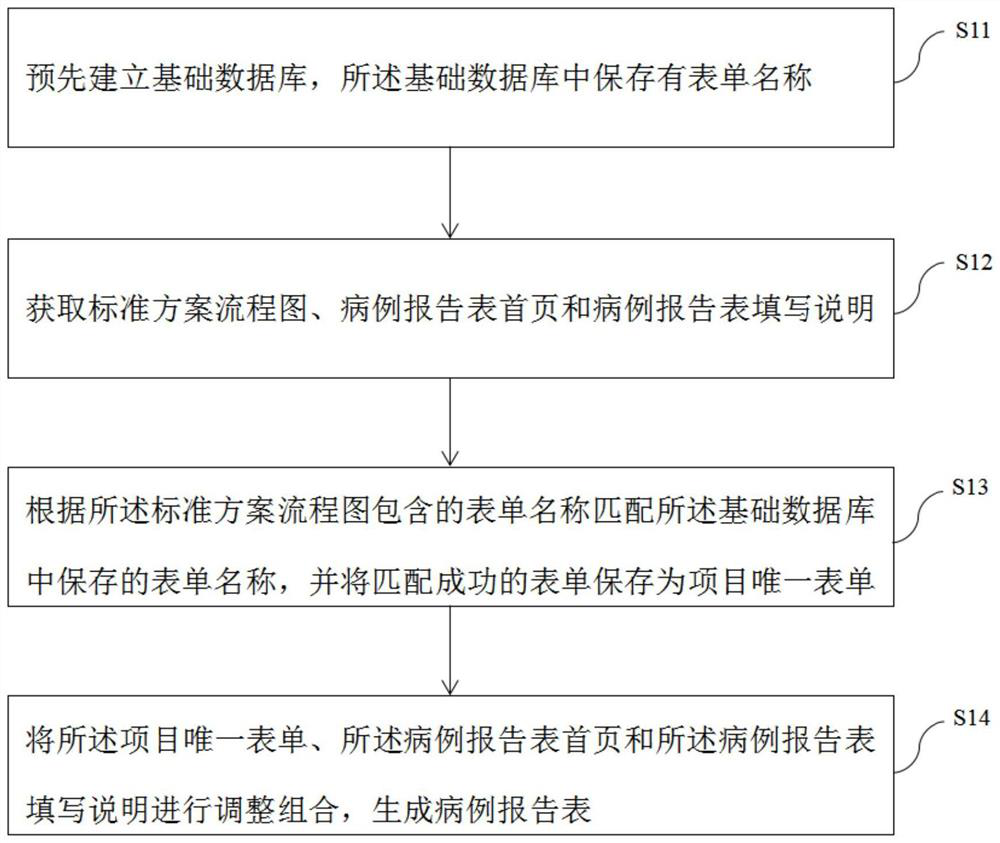
Clinical test case report form automatic generation method, device and equipment
PendingCN114300084ACorrectly designedComplete efficientlyDigital data information retrievalText processingHome pageSoftware engineering
The invention relates to the technical field of clinical trials, in particular to a clinical test case report form automatic generation method, device and equipment, and the method comprises the following steps: firstly establishing a basic database in advance, storing a form name in the basic database, and then obtaining a standard scheme flow chart, a case report form home page and a case report form filling description, according to the form name of the standard scheme flow chart, the form name stored in the basic database is matched, the successfully matched form is stored as a project unique form, and finally, the project unique form, the case report form home page and the case report form fill in description adjustment combinations to generate a case report form. According to the method, the case report form can be directly and automatically generated on the basis of the clinical test scheme, so that the design of the clinical test case report form can be accurately and efficiently completed.
Owner:CLINDATA PHARM BIOTECHNOLOGY (BEIJING) CO LTD
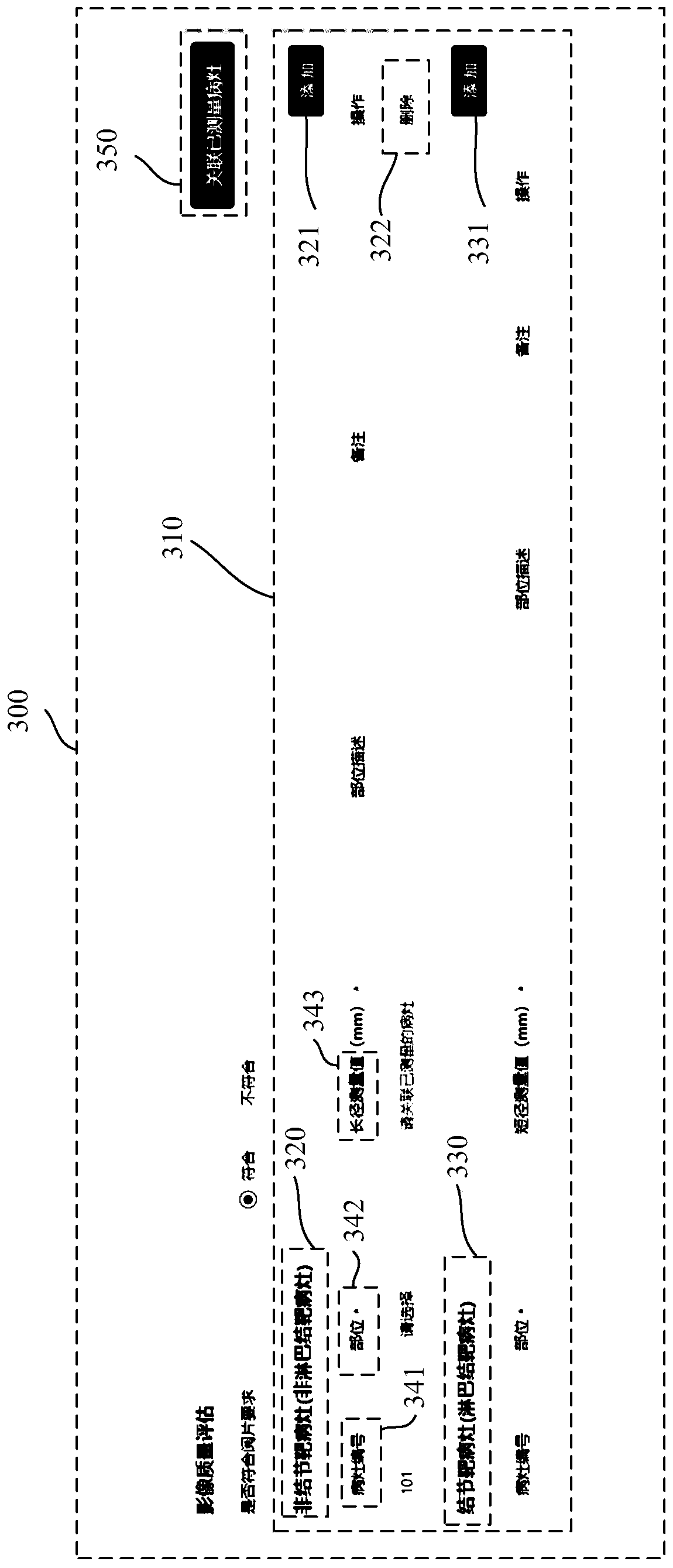
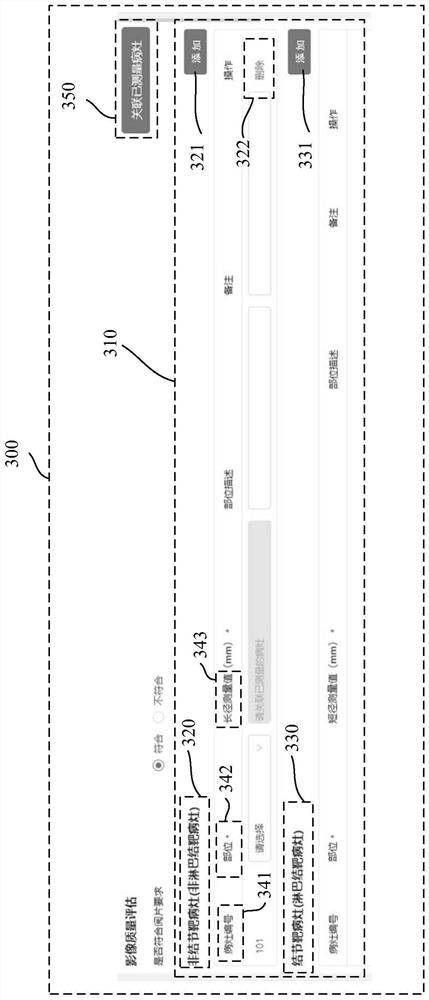
Medical image reading interaction method and system and computer readable medium
ActiveCN111142758AImprove accuracyImprove unityMedical reportsInput/output processes for data processingLesion NumberEngineering
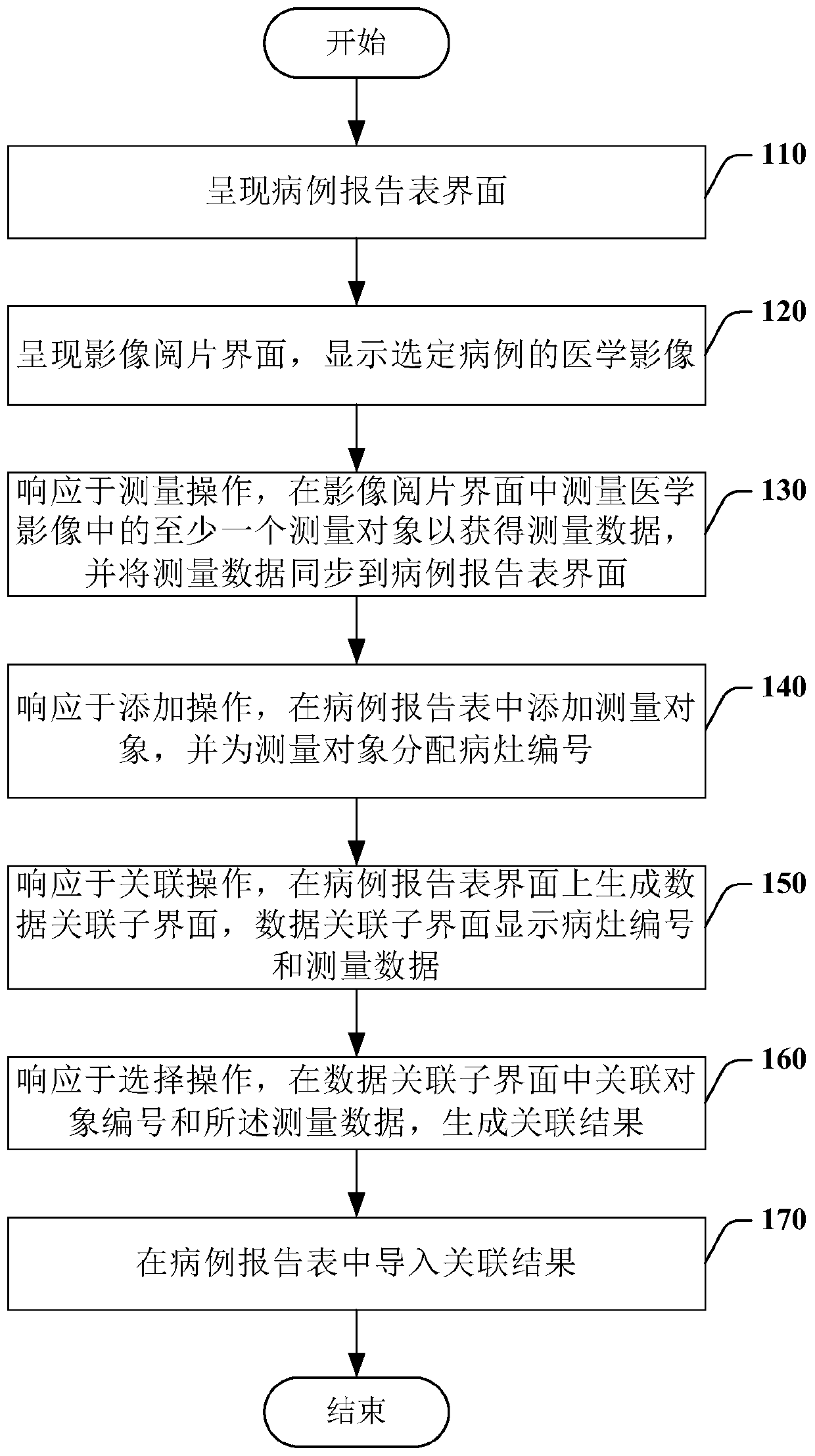
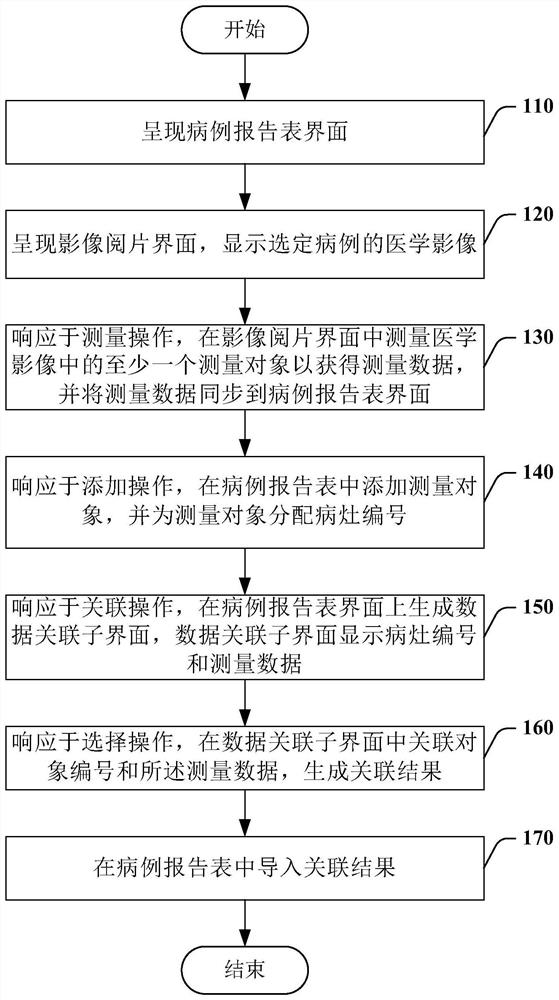
The invention relates to a medical image reading interaction method, and the method comprises the steps of presenting a case report form interface, wherein the case report form interface comprises a case report form of a selected case; presenting an image reading interface, and displaying the medical image of the selected case; in response to a measurement operation, measuring at least one measurement object in the medical image in the image reading interface to obtain the measurement data, and synchronizing the measurement data to the case report form interface; in response to an addition operation, adding a measurement object in the case report table, and allocating a focus number to the measurement object; in response to an association operation, generating a data association sub-interface on the case report form interface, and displaying the lesion number and the measurement data on the data association sub-interface; in response to a selection operation, associating the focus number and the measurement data in the data association sub-interface, and generating an association result; and importing the association result into the case report table.
Owner:上海太美星云数字科技有限公司
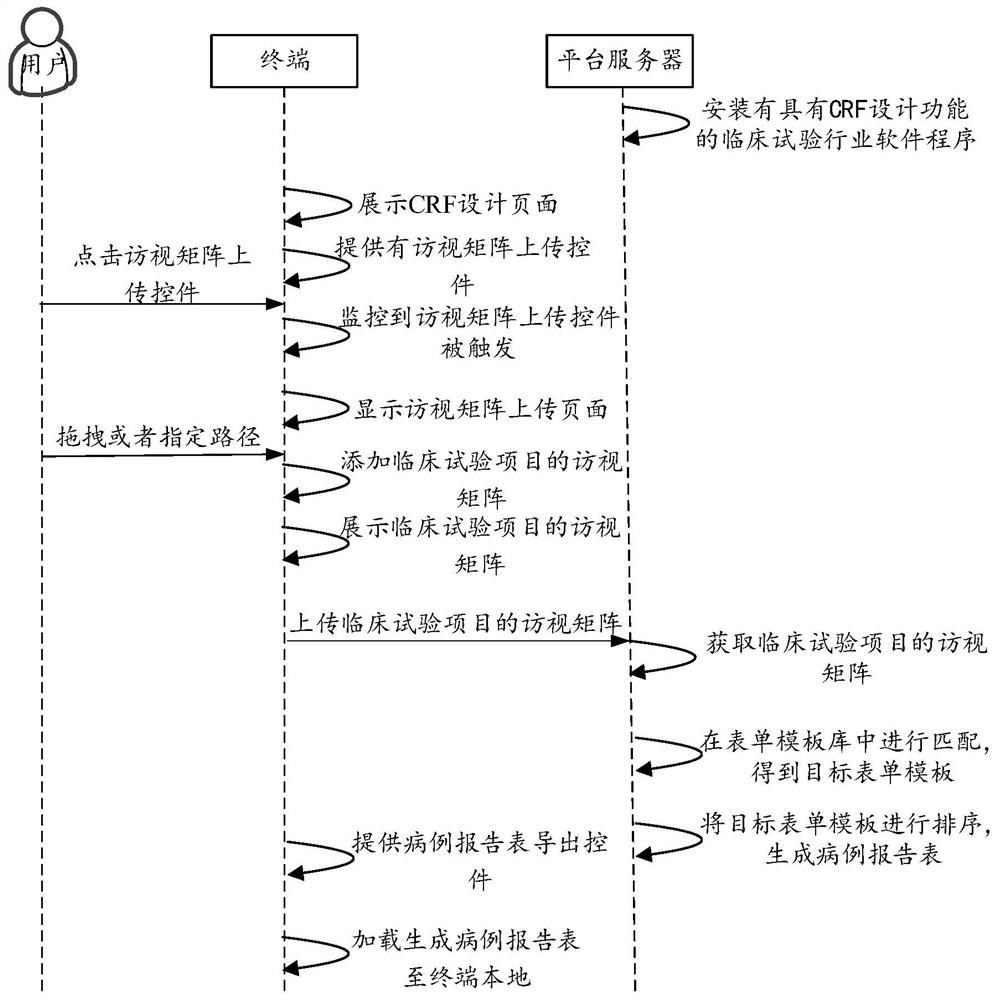
Case report form generation method and device, computer equipment and storage medium
PendingCN114005505AImprove design efficiencyReduce costs involvedText processingMedical reportsSoftware engineeringClinical tests
The invention provides a case report form generation method and device, computer equipment, a storage medium and a computer program product. The method comprises the following steps: acquiring a visit matrix of a clinical test item; matching in a form template library by using the form name in the visit matrix to obtain a target form template corresponding to the form name; and finally, sorting the target form templates based on the sequence of the visit in the visit matrix, and generating the case report form. The case report form is automatically generated based on the obtained visit matrix, the design efficiency of the case report form is improved, the related cost of the case report form is reduced, and the problem that in the prior art, a CRF design tool is used for generating CRF, and huge labor cost and time cost are consumed is solved.
Owner:浙江太美医疗科技股份有限公司
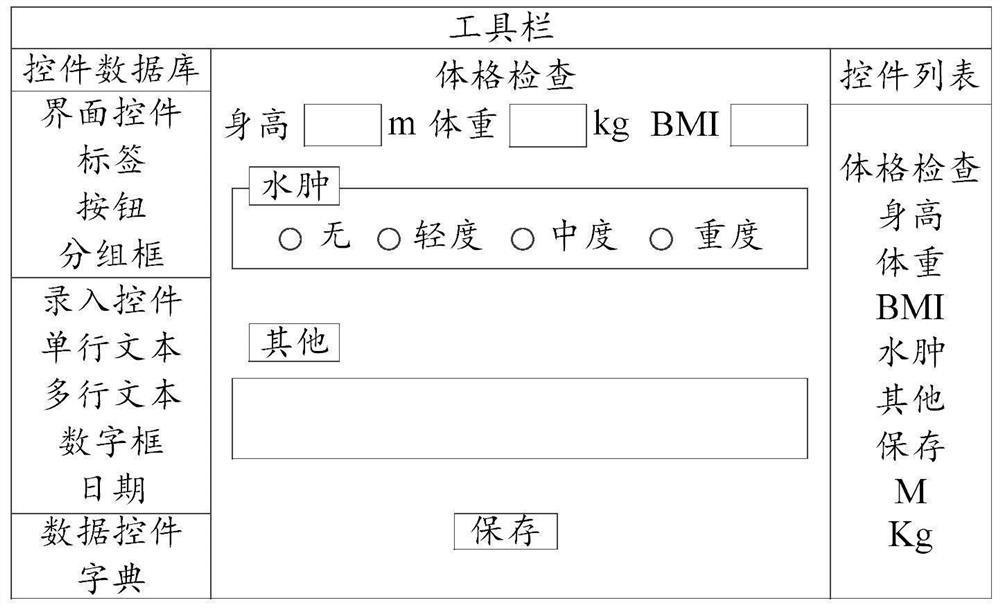
Medical record form designer and target form interface generation method
PendingCN113238750AEasy to operateData research is convenientSoftware engineeringText processingMedical recordFeature extraction
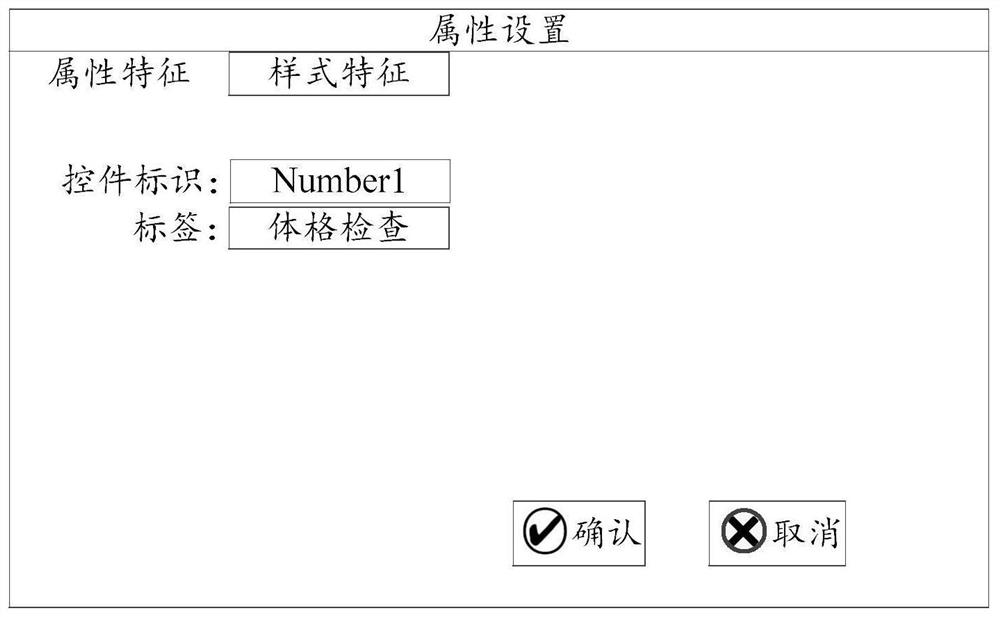
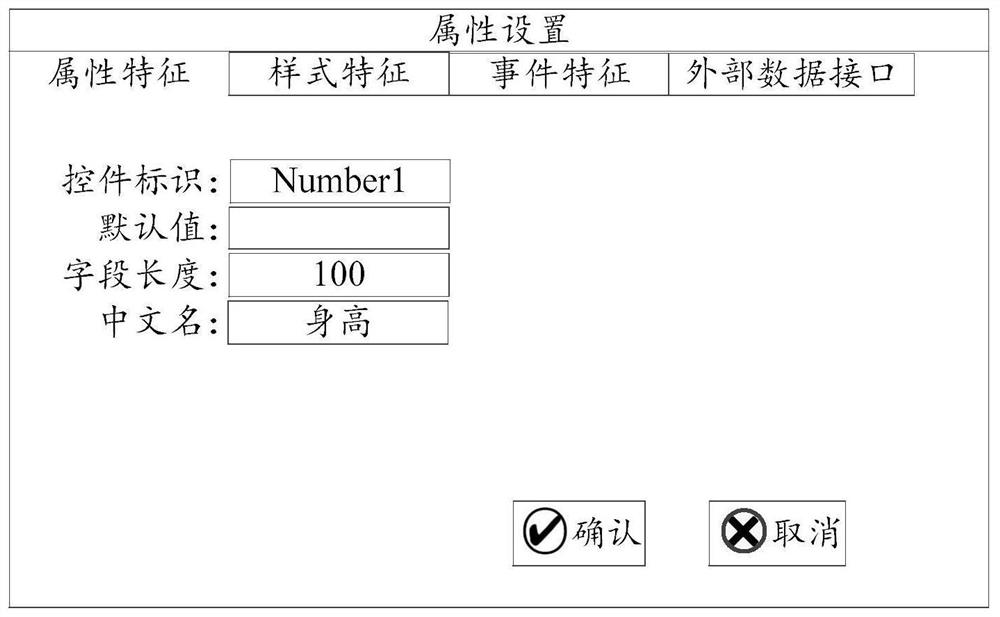
The embodiment of the invention provides a medical record form designer and a target form interface generation method. The medical record form designer comprises: a target form interface generation unit, a form control library, a form control attribute setting unit and a form control list unit. According to the case form designer, the form controls corresponding to the field features are extracted according to the field features of the to-be-detected items in the original case report form, the target form interface is generated according to the form control corresponding to at least one field feature, and the form controls of the target form interface comprise control attribute information. The control attribute information comprises a function for representing that the form control has information interaction with an external data information system. Therefore, the operation mode of designing the target form interface by the form designer is simple and convenient, a medical worker can conveniently design the target form interface corresponding to the principle case report form, and the generated field feature value input by the target form interface provides convenience for data research of the medical worker.
Owner:东华医为科技有限公司
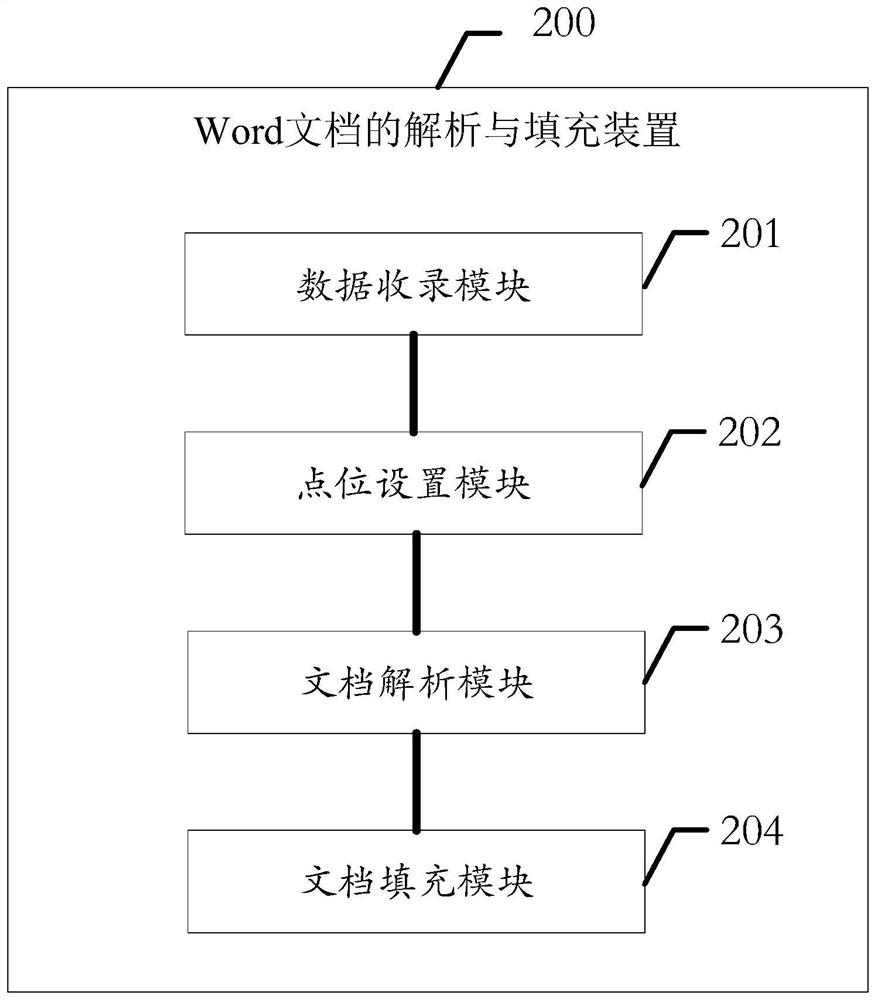
Word document analysis and filling method and device, equipment and storage medium
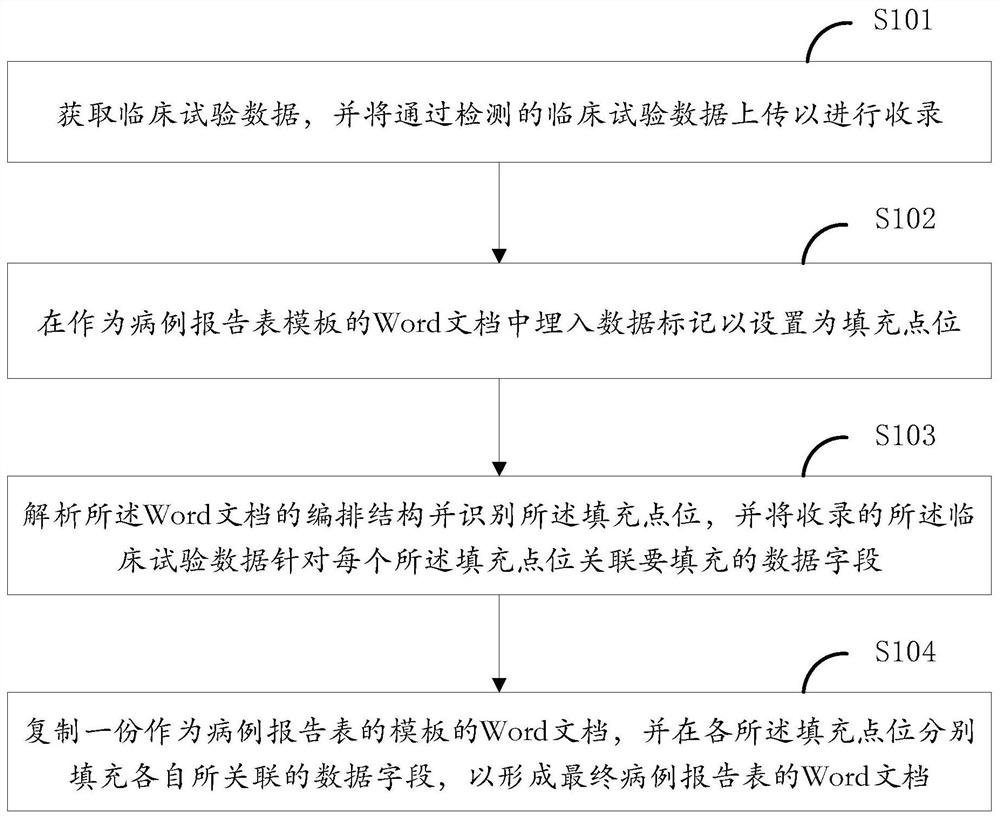
PendingCN111859899AReduce workloadEasy to manage centrallyNatural language data processingDocument analysisClinical tests
The invention provides a Word document analysis and filling method and device, equipment and a storage medium. The method comprises the steps: obtaining clinical test data, and uploading the detectedclinical test data for recording; embedding data marks into a Word document serving as a case report form template to set the data marks as filling point locations; analyzing an arrangement structureof the Word document, identifying the filling point locations, and associating the recorded clinical test data with data fields to be filled for each filling point location; and copying a Word document serving as a template of the case report form, and respectively filling the associated data fields in the filling point locations to form a final Word document of the case report form. According tothe invention, the workload of researchers can be effectively reduced, and the centralized management of scientific research data is facilitated; and meanwhile, since the word document does not need to be generated from zero, the universality is better, and the document can be randomly configured according to the requirements of researchers.
Owner:合肥森亿智能科技有限公司 +1
Time sequence analysis method and device for clinical test data, electronic equipment and medium
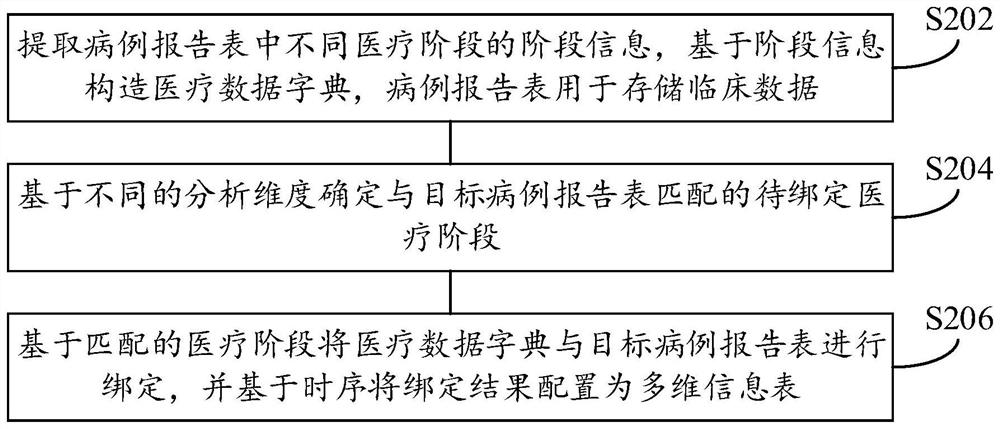
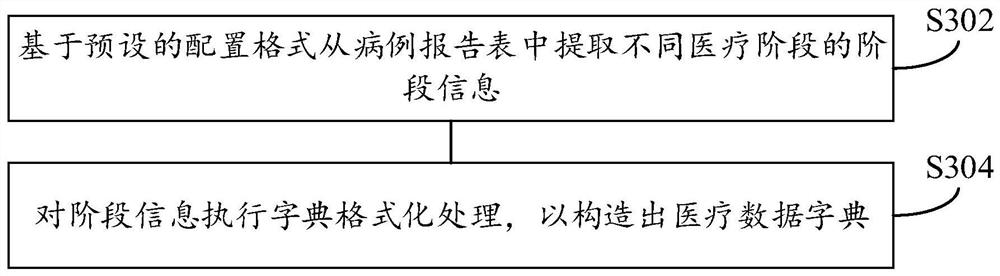
PendingCN112786126ALow cost of executionImprove securityRelational databasesMulti-dimensional databasesClinical testsEngineering
The invention provides a time sequence analysis method and device for clinical test data, electronic equipment and a computer readable storage medium, and relates to the field of data analysis. The time sequence analysis method for the clinical test data comprises the steps: extracting the stage information of different medical stages in a case report form, constructing a medical data dictionary based on the stage information, the case report form being used for storing the clinical test data; determining a to-be-bound medical stage matched with the target case report form based on different analysis dimensions; and binding the medical data dictionary with the target case report form based on the to-be-bound medical stage, and configuring a binding result as a multi-dimensional information table based on a time sequence. Through the technical scheme disclosed by the invention, the auxiliary judgment efficiency of the clinical test data on the safety and effectiveness of the clinical treatment scheme can be improved.
Owner:TIANJIN HAPPY LIFE TECH CO LTD
Method for optimizing clinical data standardization
InactiveUS9002875B2Digital data information retrievalDigital data processing detailsData setClinical trial
A method of integrating clinical trial data into a form required by a standards based data format. Annotations may be made to a case report form (CRF) designed for a clinical trial that map report form data to standards compliant data. The annotations may be stored in, and then applied to data captured using that particular case report form to produce standards compliant data for that particular clinical trial. The data sets produced may then be validated as being standards compliant.
Owner:GOPALAKRISHNAN KALYAN +2
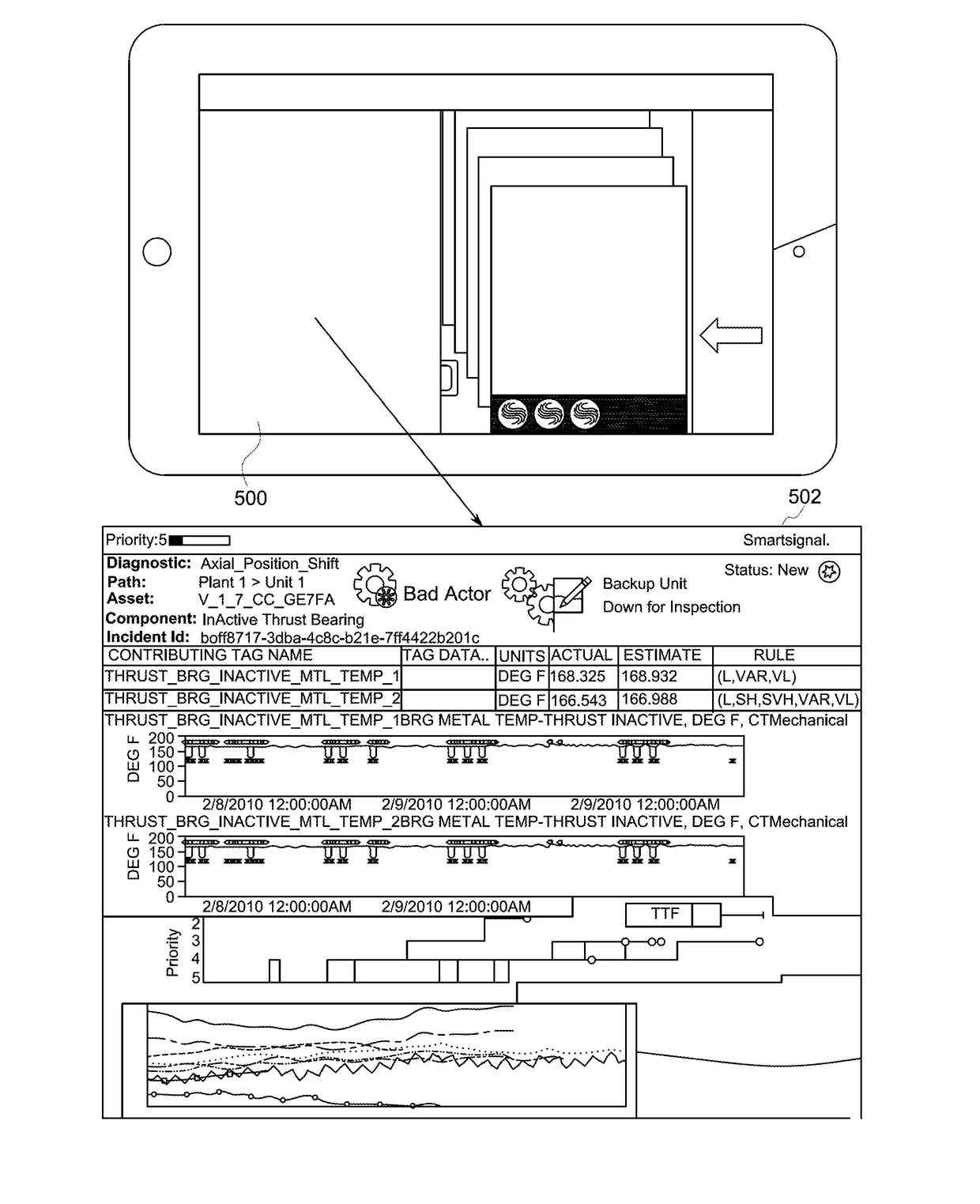
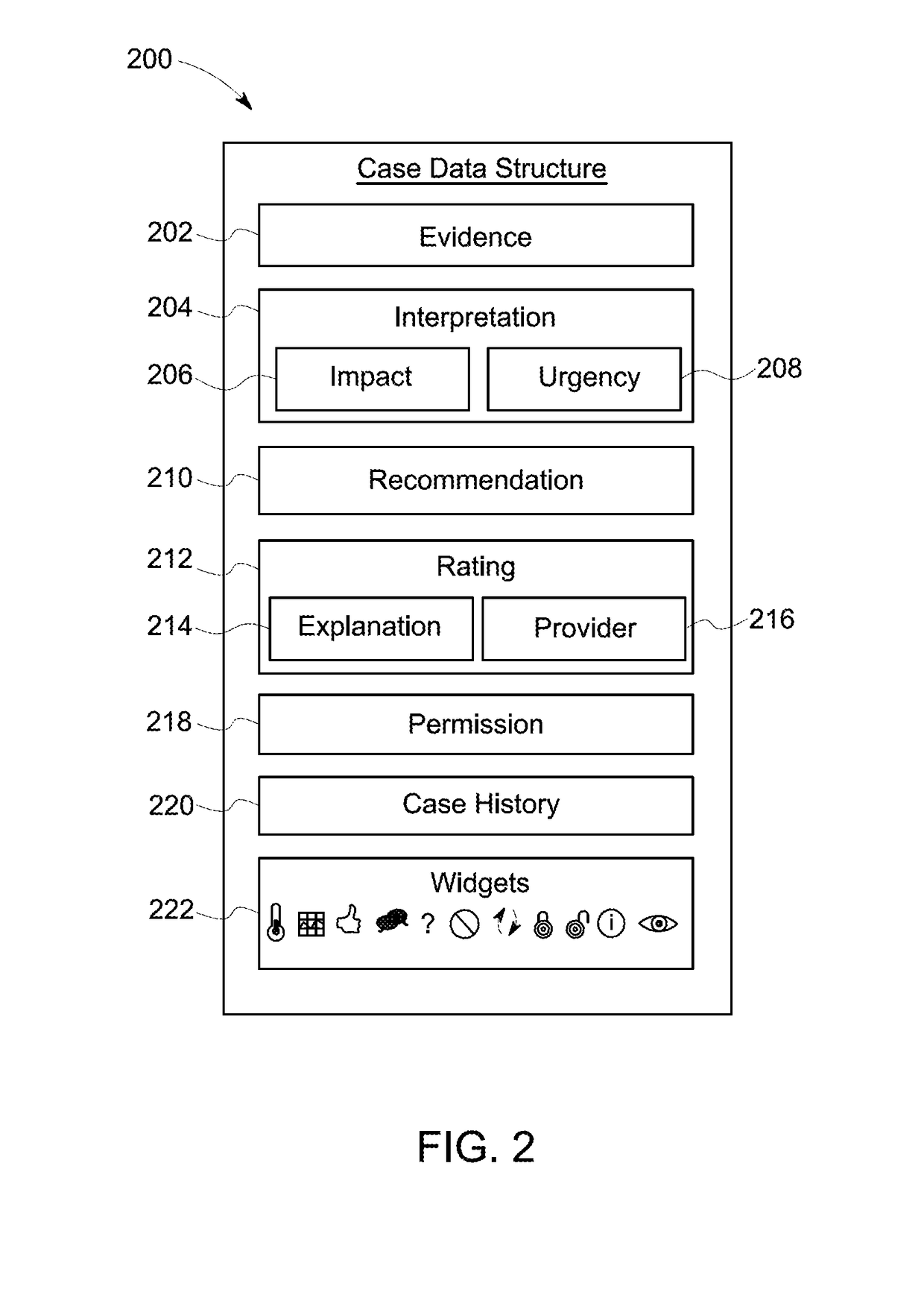
Method and apparatus for providing a multi-pane graphical display with information associated with a case data structure
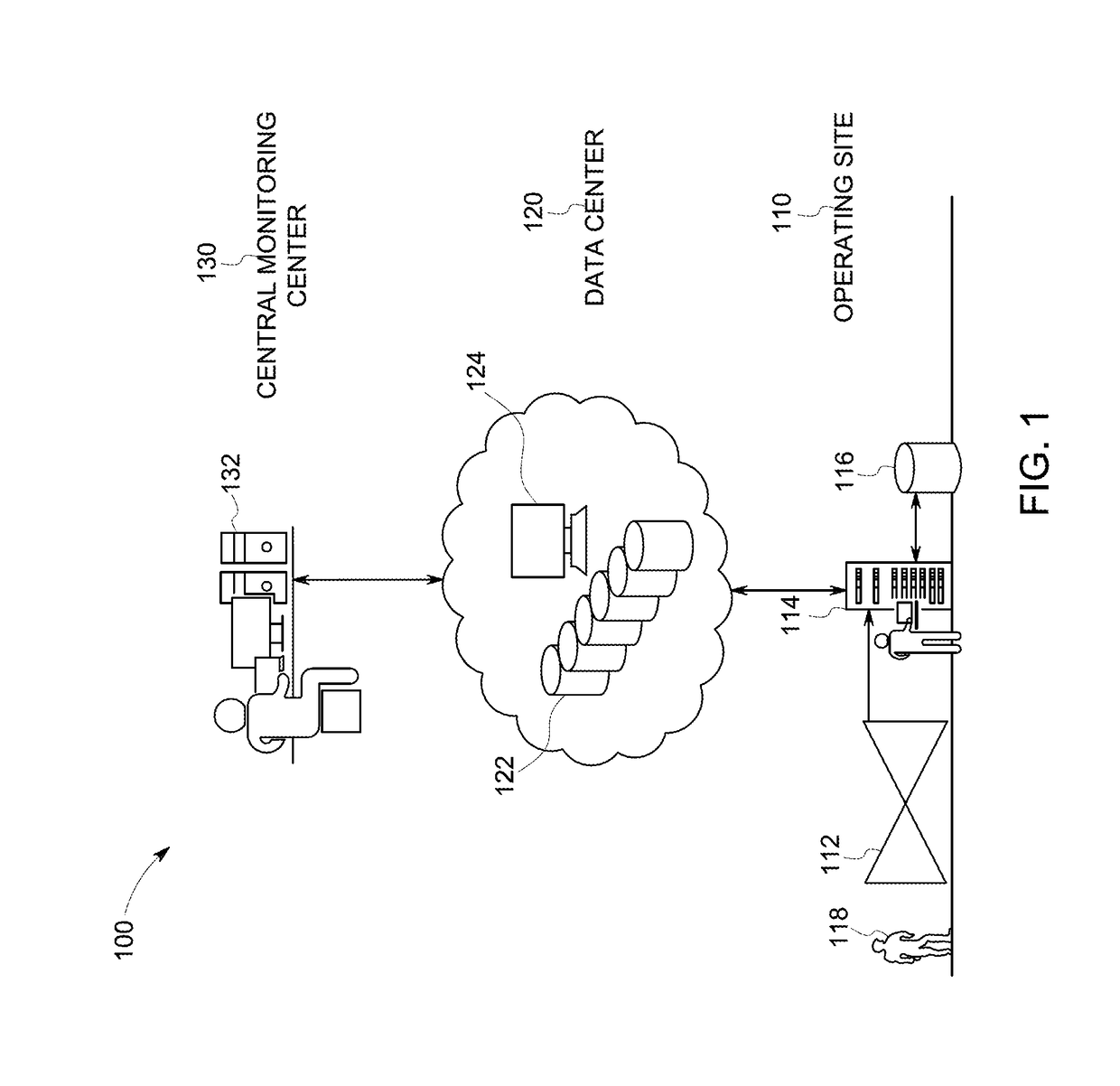
InactiveUS20170262160A1TransmissionOther databases browsing/visualisationGraphicsArray data structure
Approaches are provided for an apparatus having an interface with an input and an output. The apparatus also includes a display device having a touch-sensitive user interface. The apparatus also includes a processor coupled to the interface and the display device. The processor is configured to display at the display device a first content pane, a second content pane, and a third content pane. The first content pane includes a case report received at the input of the interface. The case report represents characteristics of a case associated with an abnormality detected in an industrial machine or system. The second content pane includes a user input interface configured to receive a user input. The third content pane includes a response to the user input. The response is received at the input of the interface.
Owner:GENERAL ELECTRIC CO
Clinical case data collection system and method
ActiveCN105468929AImprove recognition accuracyRecognition speed is fastSpecial data processing applicationsPatient-specific dataComputer moduleData acquisition
The invention relates to a clinical case data collection system and method. A verification module of a recognition device comprises an electronic case report form comparison unit. The electronic case report form comparison unit receives a first electronic case report form sent by a first OCR module and a second electronic case report form sent by a second OCR module and compares the electronic case report forms sent by the first OCR module and the second OCR module. In this way, while the working efficiency of converting paper case report forms into electronic case report forms is improved, output of mistaken electronic case report forms is effectively reduced, and the accuracy and speed of the clinical case data collection system are improved. Moreover, the first OCR module and the second OCR module recognize the paper case report forms respectively through different algorithms, and therefore the accuracy of comparison between the first electronic case report form and the second electronic case report form through the electronic case report form comparison unit can be enhanced.
Owner:CHINESE ACAD OF PREVENTIVE MEDICINE
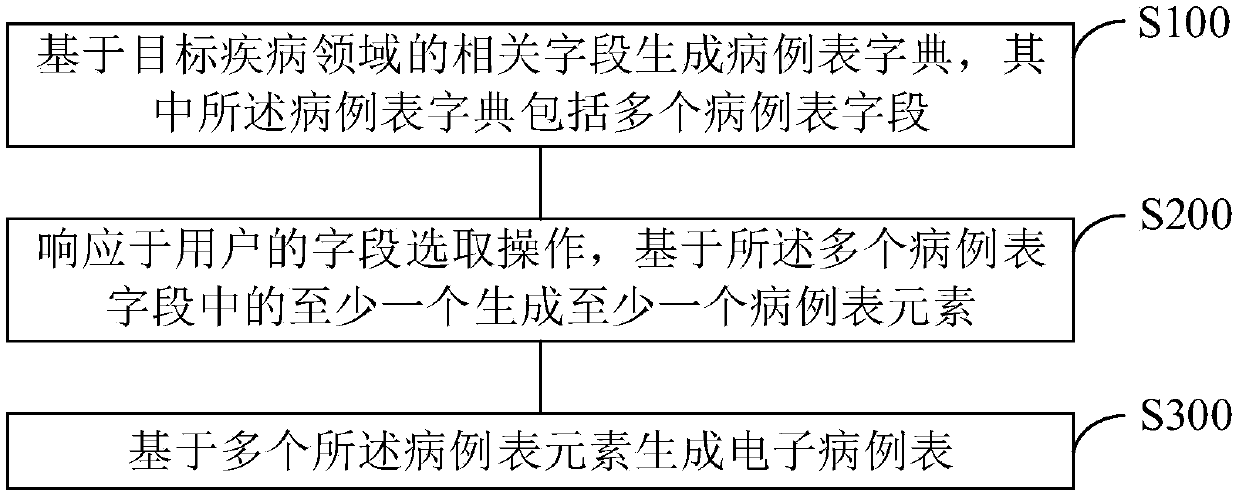
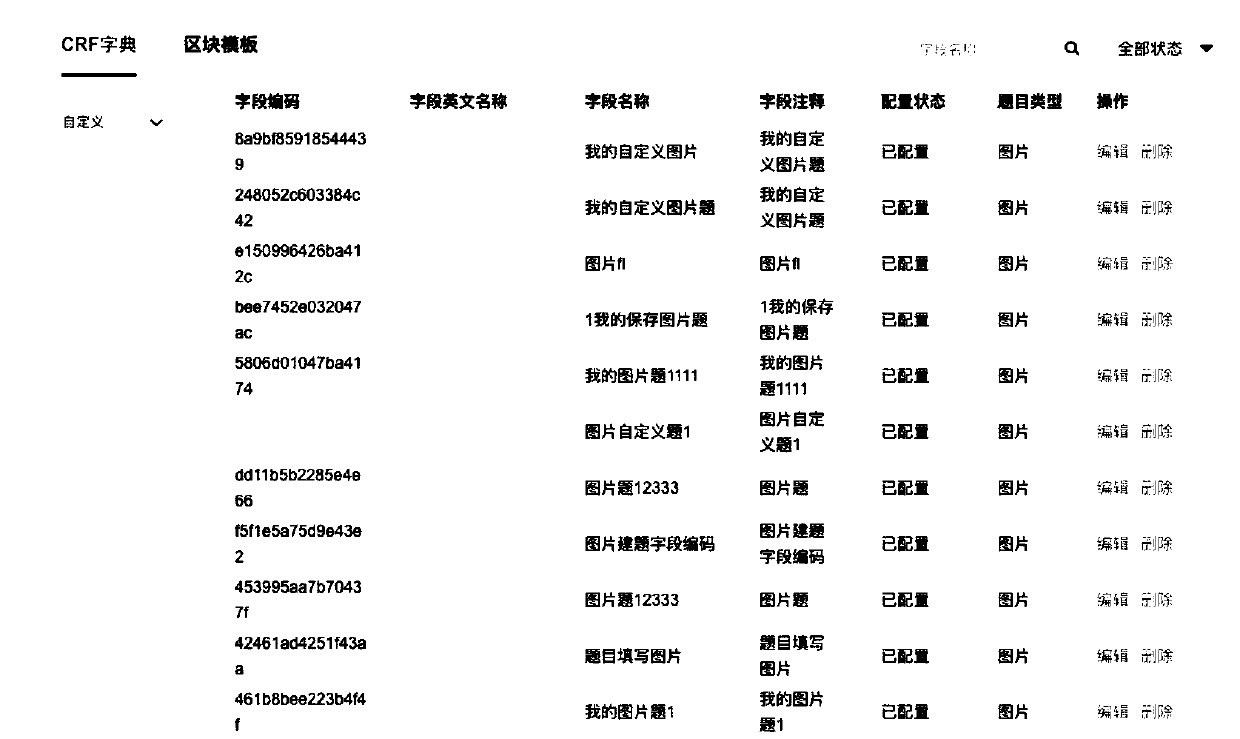
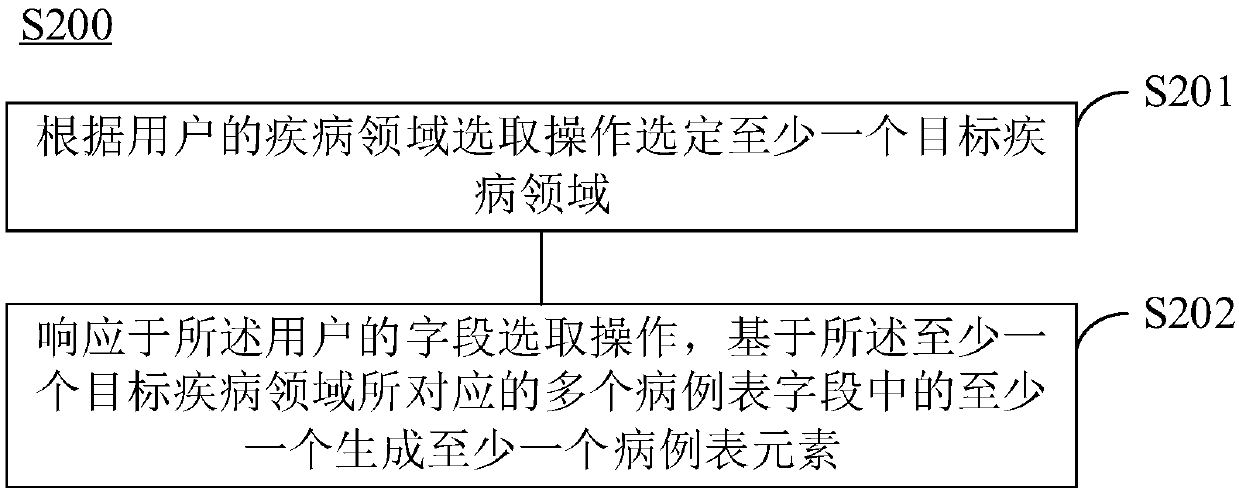
Electronic case form generating method and device
PendingCN109256182AReduce dependenceEliminate workloadText processingSpecial data processing applicationsDiseaseData mining
The present invention provides an electronic case form generating method and device. The method includes the following steps that: a case form dictionary is generated according to related fields in atarget disease field, wherein the case form dictionary includes a plurality of case form fields; at least one case form element is generated on the basis of at least one of the plurality of case formfields in response to the field selection operation of a user; and an electronic case form is generated on the basis of the plurality of case form elements. With the electronic case form generating method and device of the invention adopted, human resource investment in the use and maintenance of electronic case report forms can be significantly decreased, and the application of an electronic dataacquisition system can be benefitted.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
Method and system for analyzing and constructing case report form
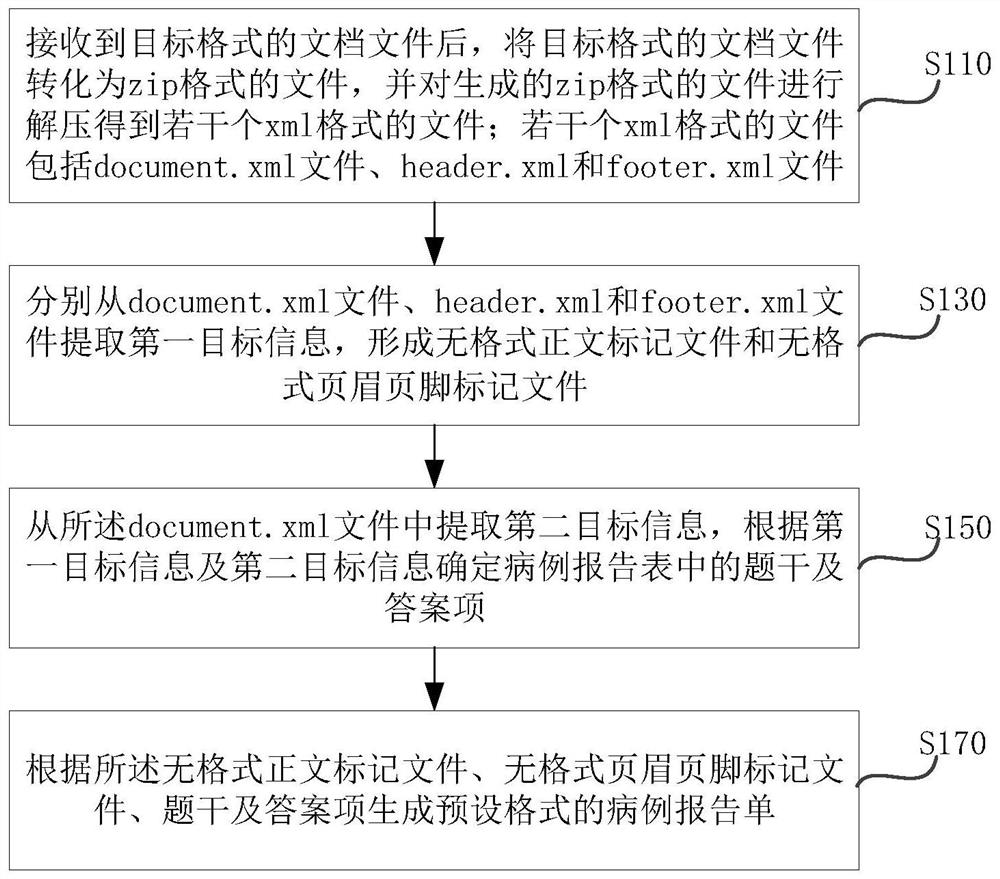
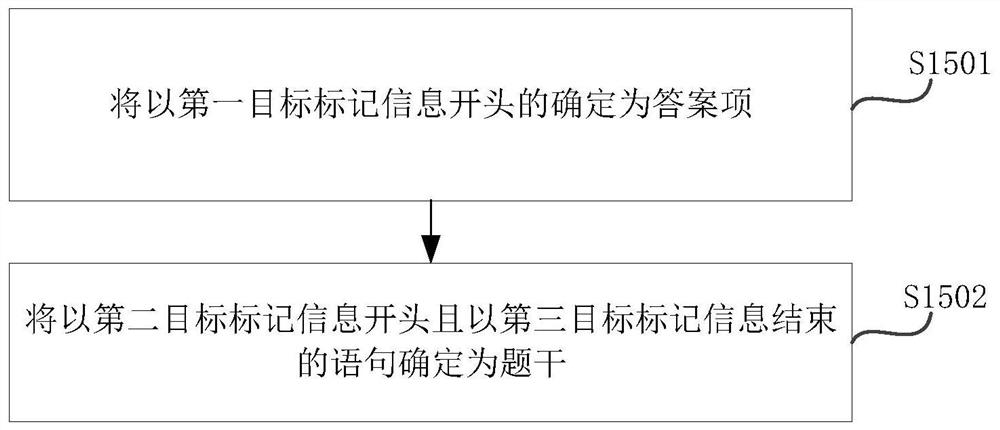
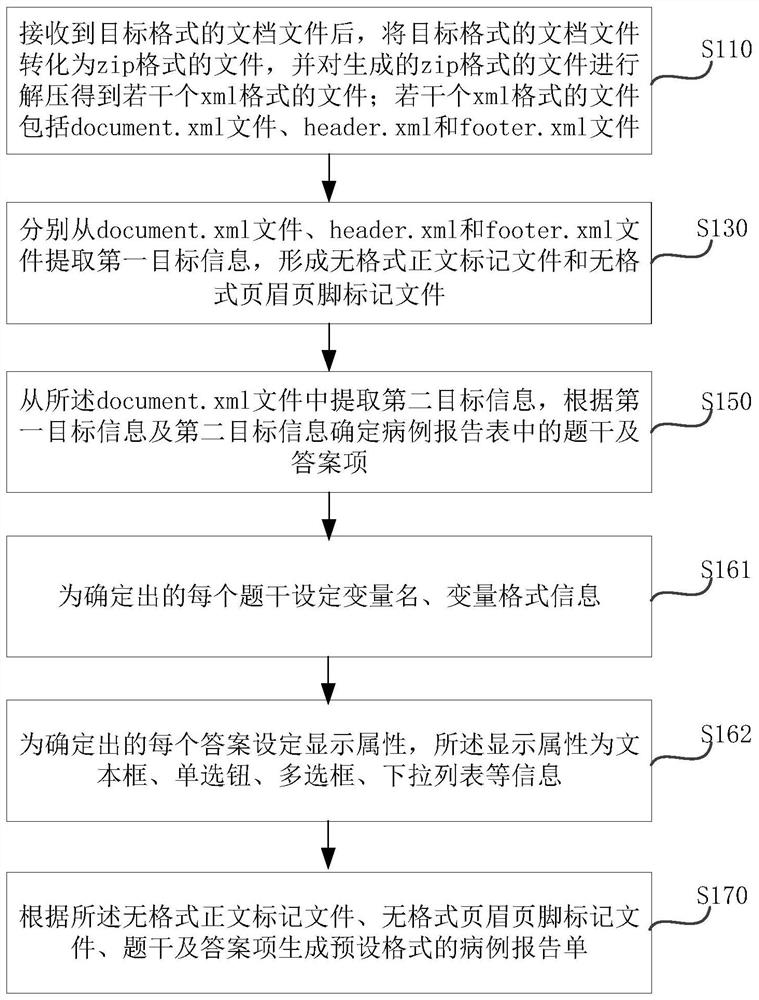
PendingCN113782135AEasy to unloadEasy to parseText processingMedical reportsEngineeringCase report form
The invention relates to the technical field of medical big data, in particular to a method and system for analyzing and constructing a case report form; the method comprises the following steps: after receiving a document file in a target format, converting the document file in the target format into a file in a zip format, and decompressing the generated file in the zip format to obtain a plurality of files in xml formats, wherein the plurality of files in the xml format comprise a document.xml file, a header.xml file and a footer.xml file; then extracting the first target information from the document.xml file, the heder.xml file and the footer.xml file respectively so as to form a formattless text marking file and a formattless header and footer marking file. The defect that in the prior art, analysis and construction of a case report form are inconvenient can be overcome.
Owner:INST OF INFORMATION ON TRADITIONAL CHINESE MEDICINE CACMS
Medical image reading interactive method, system and computer readable medium
ActiveCN111142758BImprove accuracyImprove unityMedical reportsInput/output processes for data processingLesion NumberEngineering
The invention relates to a medical image reading interaction method, and the method comprises the steps of presenting a case report form interface, wherein the case report form interface comprises a case report form of a selected case; presenting an image reading interface, and displaying the medical image of the selected case; in response to a measurement operation, measuring at least one measurement object in the medical image in the image reading interface to obtain the measurement data, and synchronizing the measurement data to the case report form interface; in response to an addition operation, adding a measurement object in the case report table, and allocating a focus number to the measurement object; in response to an association operation, generating a data association sub-interface on the case report form interface, and displaying the lesion number and the measurement data on the data association sub-interface; in response to a selection operation, associating the focus number and the measurement data in the data association sub-interface, and generating an association result; and importing the association result into the case report table.
Owner:上海太美星云数字科技有限公司
Case report form design method and device
ActiveCN106445901BIn line with the habit of thinkingEasy to integrateText processingSpecial data processing applicationsUser inputTime cost
The disclosure relates to a method and a device for designing a case report form. The method comprises the following steps: providing multiple data options on a user interface; receiving a user input and determining the data options selected by a user according to the user input; sending a first request for acquiring attribute information of the data options selected by the user to a server, wherein the server stores attribute information of each data option; receiving the attribute information of the data options selected by the user, returned by the server in response to the first request; and automatically generating the case report form on the basis of the data options selected by the user and the received attribute information of the data options selected by the user. The method and the device provided by the disclosure can reduce the time cost and the manpower cost for designing the case report form, and are convenient for integration and statistical analysis of acquired data.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
Management method and equipment of medical scientific research follow-up task, and medium
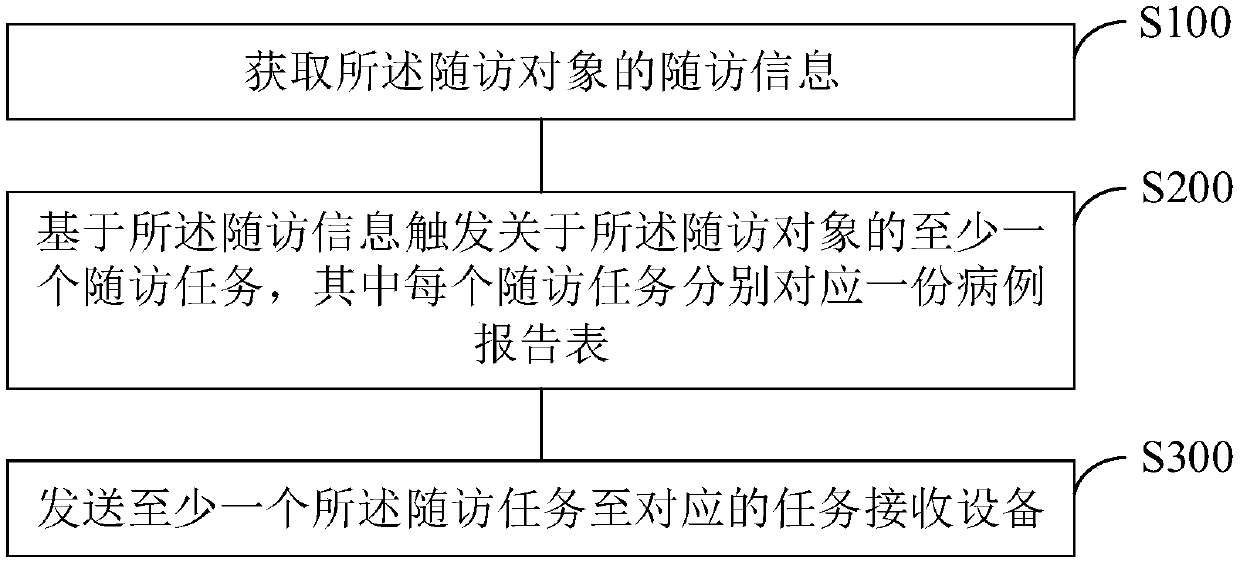
InactiveCN109545292ARealize managementTake advantage ofPatient healthcareHealthcare resources and facilitiesDiseaseObject based
The application provides a management method and equipment of a medical scientific research follow-up task, and a medium. The method comprises the following steps: acquiring follow-up information of afollow-up object, and triggering at least one follow-up task about the follow-up object based on the follow-up information, wherein each follow-up task corresponds to one case report form; sending atleast one follow-up task to the corresponding task receiving equipment, wherein each follow-up task comprises the own follow-up performing time. Through the management method provided by the application, the existing patient data in the disease library can be sufficiently utilized, thereby helping the follow-up visitor to trigger the follow-up beginning by automatically judging whether the patient meets the requirement of the follow-up plan, and reducing the follow-up lose rate.
Owner:YIDU CLOUD (BEIJING) TECH CO LTD
Case information data migration, storage method and system
ActiveCN112133387BSimplify complexityImprove efficiencyMedical reportsInstrumentsData miningCase report form
Owner:杭州太美星程医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com