Clinical test case report form automatic generation method, device and equipment
A technology for case report forms and clinical trials, which is applied in the field of automatic generation of clinical trial case report forms, can solve the problems of low processing efficiency, long time-consuming, and slow progress of clinical trial projects, and achieve the effect of accurate design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
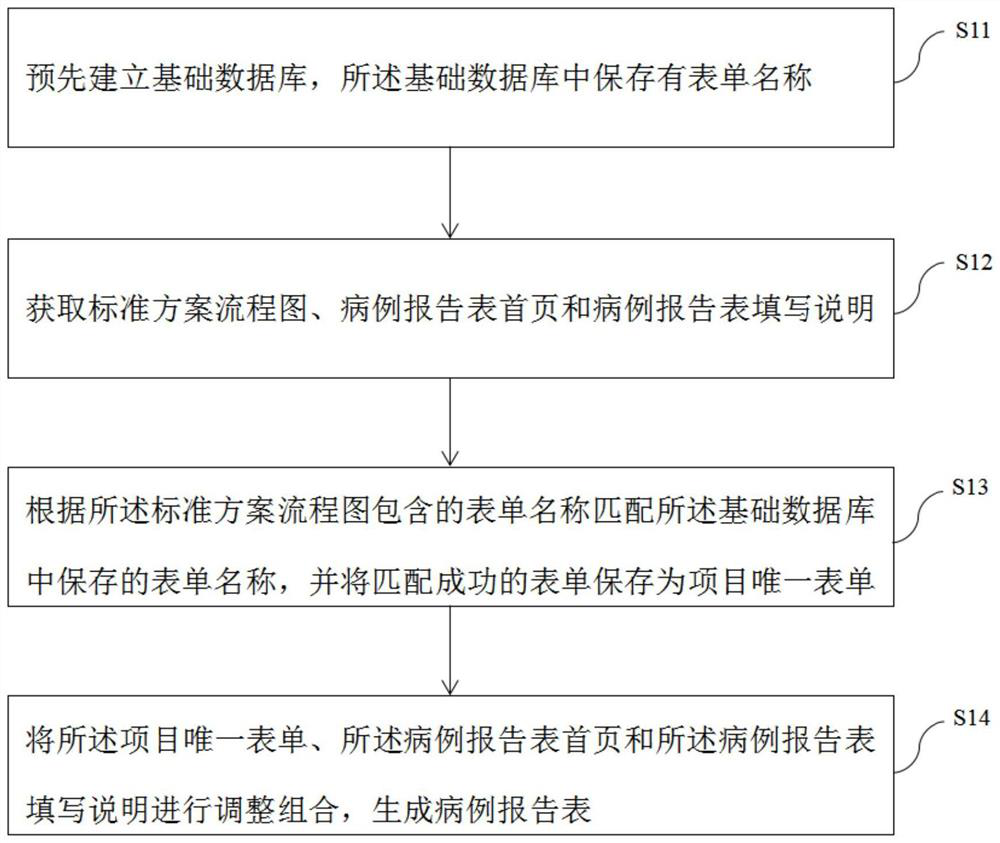
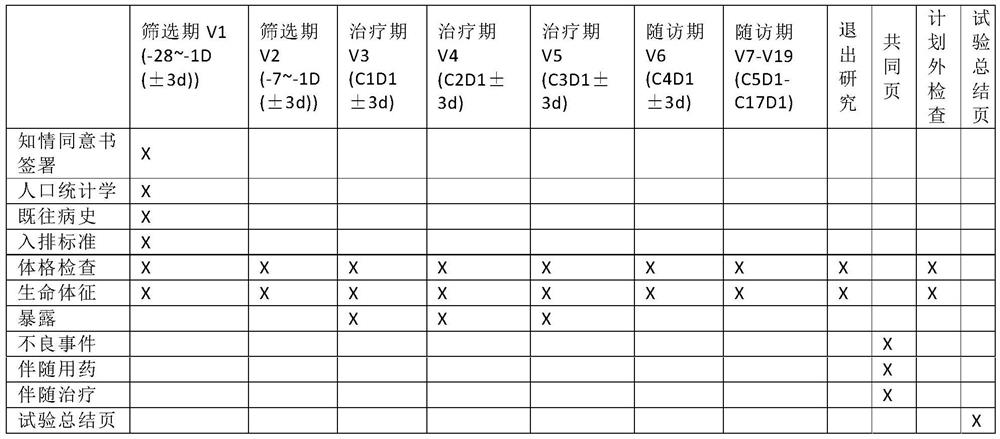
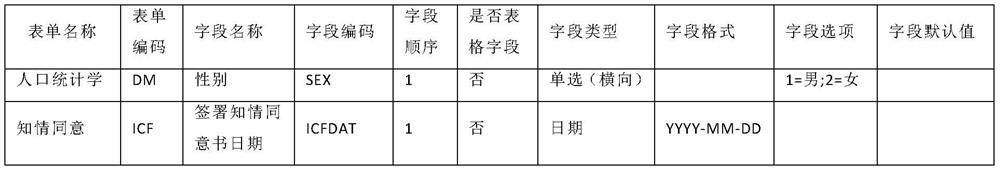
[0037] Exemplary embodiments will be described in detail herein, examples of which are illustrated in the accompanying drawings. Where the following description refers to the drawings, the same numerals in different drawings refer to the same or similar elements unless otherwise indicated. The implementations described in the illustrative examples below are not intended to represent all implementations consistent with this application. Rather, they are merely examples of apparatus and methods consistent with some aspects of the present application as recited in the appended claims.
[0038] In order to solve the above problems, the present application provides a method, device and equipment for automatically generating a clinical trial case report form, which is used to fill in the gaps in the prior art, thereby shortening its design cycle, reducing the time of clinical trials, and improving the quality of clinical trials. , reduce the cost of clinical trials. The specific i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com