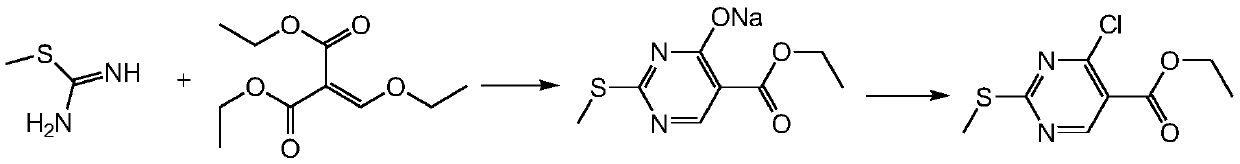
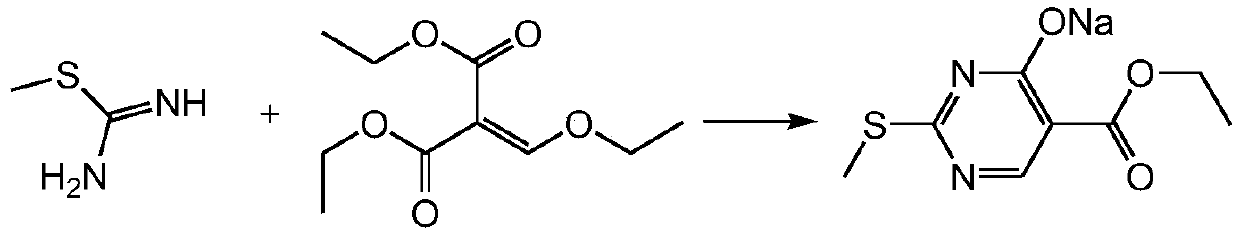
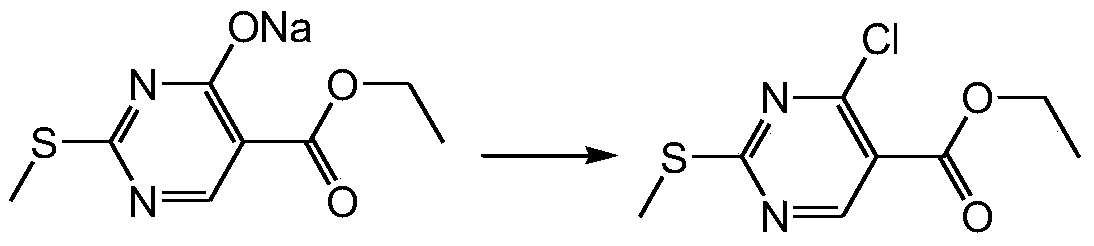
Method for synthesizing ethyl 4-chloro-2-methylthiopyrimidine-5-carboxylate
A technology of methylthiopyrimidine and ethyl formate, applied in the field of synthesis of 4-chloro-2-methylthiopyrimidine-5-ethyl carboxylate, which can solve the problems of poor reaction conditions, difficult operation, product decomposition and deterioration, etc. problems, to achieve the effects of cost and productivity improvement, reaction safety improvement, simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] first step
[0024]
[0025] Into a clean 20L reaction bottle, add 5kg of water and 5kg of ethanol, and stir evenly. Take 1.8kg of solid sodium hydroxide, add it into the system and stir for 30min. until it is completely dissolved. Cool down to below 25°C in an ice bath, add 3.2 kg of S-methylisothiouronium sulfate, basically no heat release, and a white turbid liquid. After addition, stir for 10 min. Add dropwise the ethanol solution of ethoxymethylene (3.88kg ethoxymethylene + 1.5kg ethanol), exothermic at the initial stage of dropping, keep the temperature below 30°C, basically no heat release later, and the dropping speed can be accelerated. After dropping, the temperature was raised to 65-70°C for 1.5h. The reaction solution was slowly poured into 10 kg of ice water while it was hot, and stirred for 20 min. Centrifuge the treatment liquid in a centrifuge to obtain 4.6kg of wet product. Air-dried at 70-75°C until constant weight. 3.6 kg of off-white solid w...
Embodiment 2
[0027] Add 150kg of water and 150kg of ethanol to a clean 500L reactor, and stir evenly. Take 54kg of solid sodium hydroxide, add it into the system and stir for 60min. until it is completely dissolved. The temperature was lowered to below 25 degrees in an ice bath, and 96 kg of S-methylisothiouronium sulfate was added, basically no heat release, and it was a white turbid liquid. After addition, stir for 30min. Add dropwise the ethanol solution of ethoxymethylene (116.5kg ethoxymethylene + 45kg ethanol), the initial stage of dropwise exothermic, heat preservation below 30 ℃, basically no exothermic later, can speed up the rate of addition. After dropping, the temperature was raised to 65-70°C for 2 hours. The reaction solution was slowly poured into 300 kg of ice water while it was hot, and stirred for 90 min. Centrifuge the treatment liquid in a centrifuge to obtain 135kg of wet product. Air-dried at 70-75°C until constant weight. 105 kg of off-white solid was obtained, ...
Embodiment 3
[0029] second step
[0030]
[0031] Into a clean 20L reaction bottle, add 3.3kg of the intermediate of the first step, 8kg of toluene, and 241g of DMF, and it becomes a cloudy liquid. Add 1200g of thionyl chloride dropwise at room temperature, 1 / 2 of the dropwise exotherm is exothermic, and there is basically no exotherm afterwards, so the dripping can be accelerated. After dropping, stir at 30°C for 30min. Take 1550 g of phosphorus oxychloride and add it dropwise to the reaction solution. Turn on the heating, slowly heat to about 60°C, a large amount of gas is generated, keep warm at 55-60°C and stir for 30min. When heated to about 65°C, the reaction solution gradually changed from turbidity to yellow clear solution. After reacting for 1 h, TLC detected that the reaction of the raw materials was complete. The reaction liquid is lowered to room temperature, slowly poured into 5kg of ice water in batches, it will release heat, preferably not higher than 20°C. Extract w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com