Pluripotent stem cell-derived oligodendrocyte progenitor cells for the treatment of spinal cord injury
A technology of oligodendrocytes and pluripotent stem cells, which can be applied to vertebrate cells, embryonic cells, animal cells, etc., and can solve problems such as development obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0088] Other implementations of the present disclosure include the following:
[0089] CLAIMS 1. A method of improving upper extremity motor function in a human subject suffering from a traumatic spinal cord injury, the method comprising administering to the subject a therapeutically effective amount of a composition comprising an allogeneic population of human oligodendrocyte progenitor cells.
[0090] 2. The method according to 1, wherein administering the composition comprises injecting the composition into the site of the spinal cord injury.
[0091] 3. The method according to 2, wherein the composition is injected about 2-10 mm caudal to the center of the spinal cord injury.
[0092] 4. The method according to 2, wherein the composition is injected about 5 mm caudal to the center of the spinal cord injury.
[0093] 5. The method according to any one of 1 to 4, wherein the human oligodendrocyte progenitor cells can be transplanted at the site of spinal cord injury.
[00...
Embodiment 1
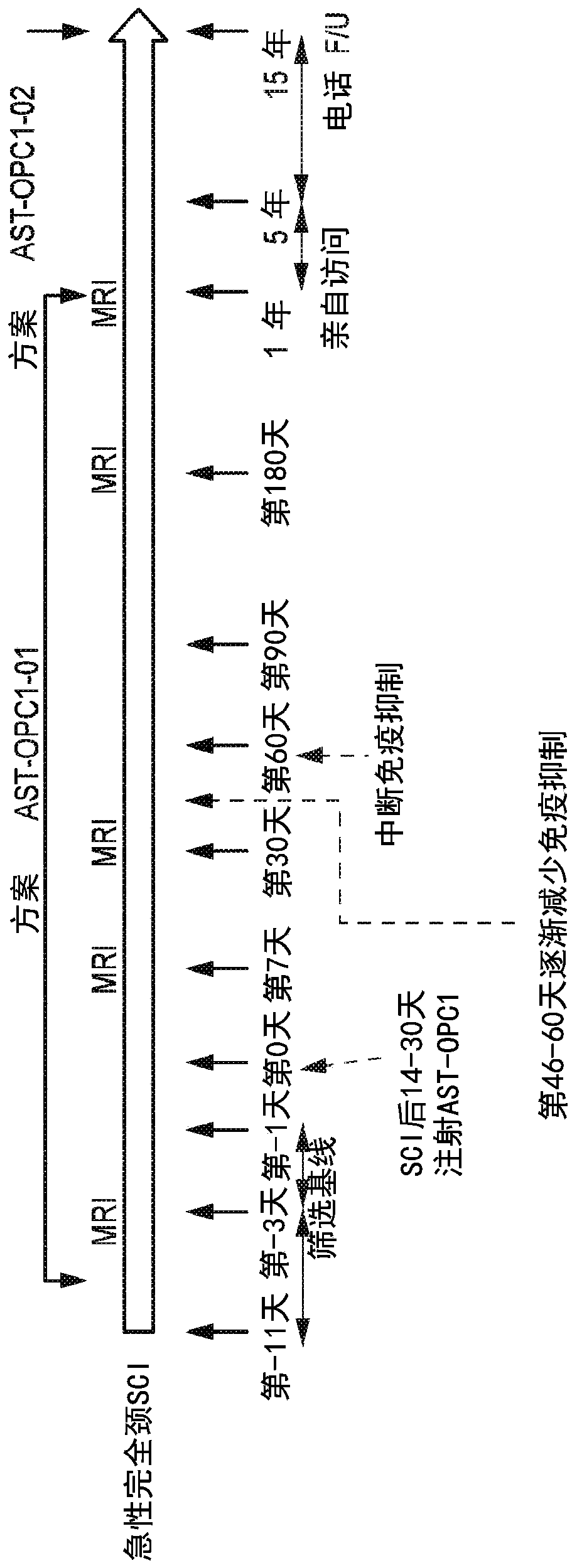
[0118] Example 1: Phase 1 / 2a Escalating Dose Trial of AST-OPC1 in Patients with Fully Motorized C4-C7 Cervical Spinal Cord Injury
[0119] AST-OPC1 cells were generated by differentiating WA01(HI) hESCs from a master cell bank (MCB) as described in US Patent Application No. 15 / 136,316.
[0120] The initial clinical safety of AST-OPC1 was previously evaluated in a Phase 1 clinical trial enrolling patients with neurologically complete T3-T11 thoracic spinal cord injury (SCI). Based on favorable 5-year safety data from the Phase 1 trial, a Phase 1 / 2a trial was initiated to evaluate the safety and activity of AST-OPC1 at elevated doses in patients with sensorimotor complete C5-C7 cervical spinal cord injury.
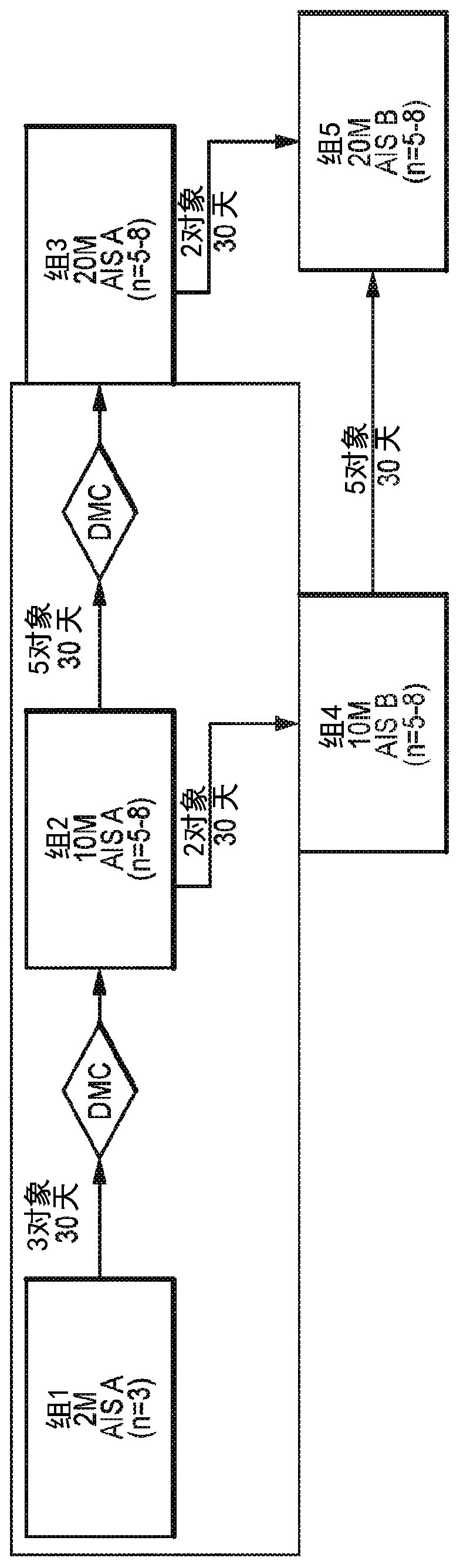
[0121] In the phase 1 trial, 5 subjects received 2 × 10 6 A dose of AST-OPC1. The Phase 1 / 2a trial has enrolled and will continue to enroll subjects to receive 2×10 between 14 and 40 days after SCI 6 , 10×10 6 or 20×10 6 A continuous dose group of AST-OPC1. The study d...
Embodiment 2
[0124] Example 2: Comparison of Upper Extremity Motor Function Improvement in Groups 1 and 2 Patients Relative to Matched Historical Controls
[0125] The motor function improvement of subjects in Groups 1 and 2 following AST-OPC1 administration was compared to a closely matched historical group of traumatic SCI patients from the EMSCI (European Multicenter Study of Spinal Cord Injury) database of over 3300 patients. The matching criteria used to generate closely matched historical control data are shown in Figure 5 .
[0126] Comparative data for the 12-month follow-up period are presented at Figure 6A , Figure 6B and Figure 7 middle.
[0127] Figure 6A : Motor function recovery measured by change in UEMS over time in Group 2 subjects (10 x 106 AST-OPC1) was beneficial compared to closely matched historical controls, with a significant improvement at 3 months and a sustained increase over the 12-month period . Figure 6B : As expected, recovery of motor function (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com