Isoquinoline derivative with hypoglycemic activity and application thereof
A technology of derivatives and isoquinoline, applied in the field of 3-aryl isoquinoline and 4-aryl quinoline derivatives and their preparation, can solve problems such as the research on non-hypoglycemic activity, and achieve good α-glucose The effect of glucosidase inhibitory activity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
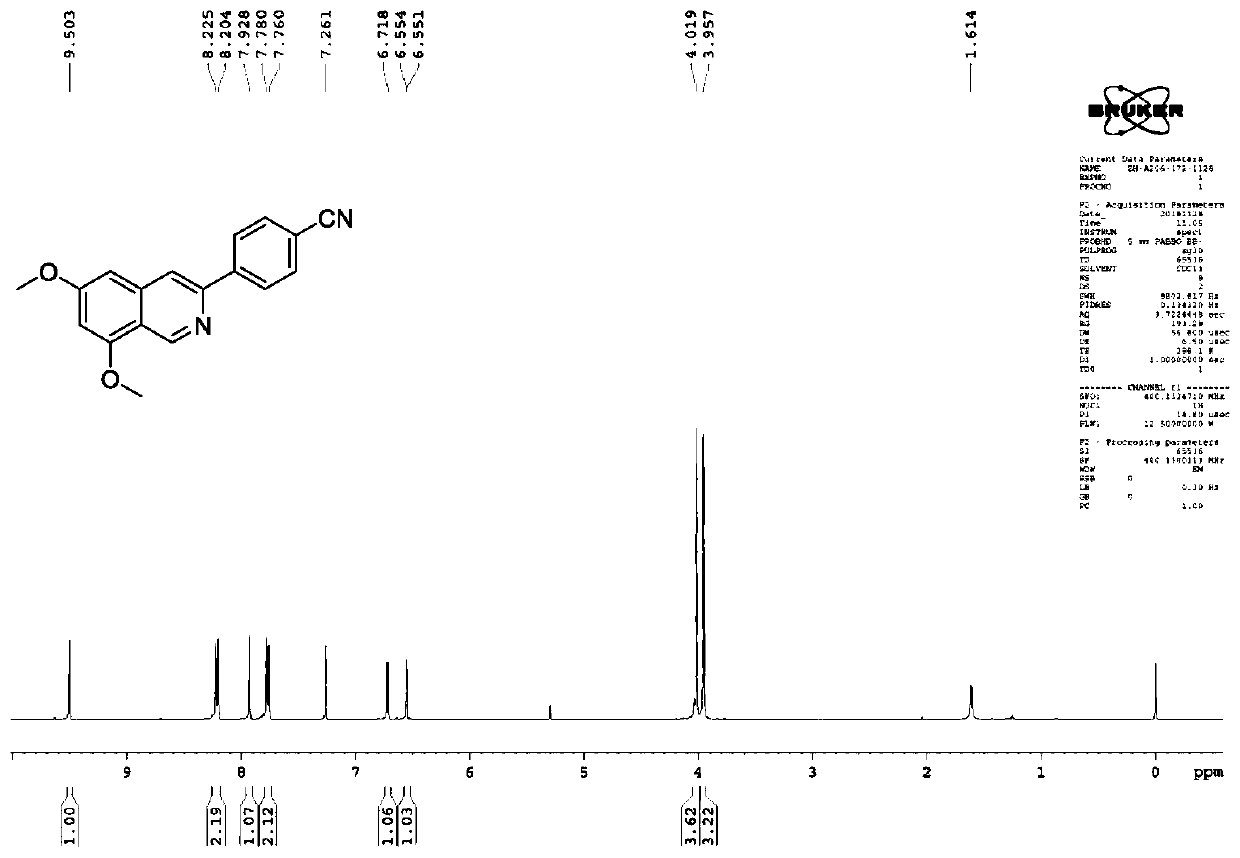
[0099] Synthesis of 4-(6,8-dimethylisoquinolin-3-yl)benzonitrile (compound 3a).
[0100]
[0101] (1) At 0°C, POCl 3 Add (1.5eq) dropwise to DMF (1.5eq), rise to room temperature after solidification, add 3,5-dimethoxyphenylacetonitrile (1.0eq), react at 65°C for 1h until the reaction solidifies into yellow, monitor the reaction after completion , with saturated NaHCO 3 Extract with ethyl acetate, collect organic phase and dry, silica gel column chromatography obtains light yellow product;
[0102] (2) Under the protection of argon, dissolve the dried product (1.0eq.) obtained in step (1) in toluene, add 4-cyanophenylboronic acid (1.2eq.), PdCl 2(dppf) (0.05eq.), cesium carbonate (3eq), react at 125°C for about 6h, after the reaction is complete, dilute with ethyl acetate, wash with water, and wash the organic layer with Na 2 SO 4 After drying and filtering, the solvent was evaporated to dryness and purified by silica gel column chromatography to obtain the product 3. ...
Embodiment 2
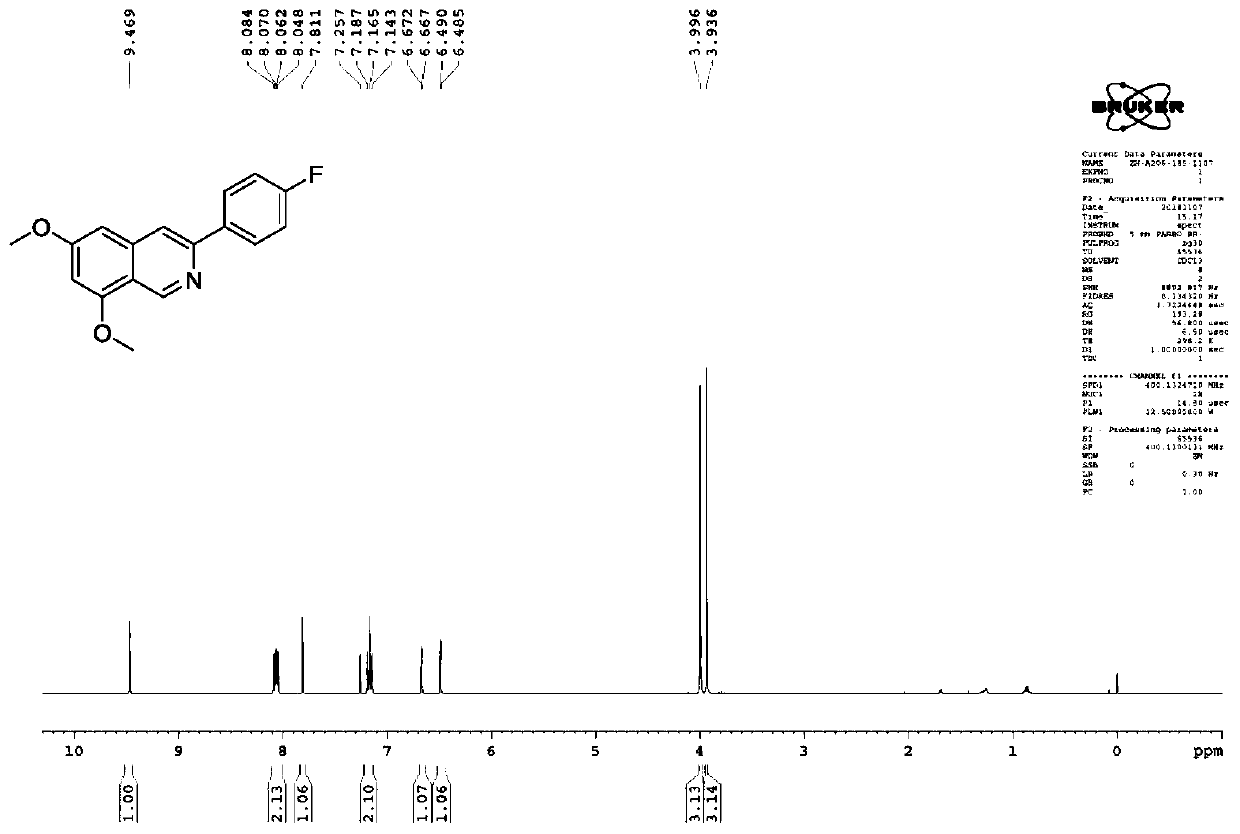
[0105] Synthesis of 3-(4-fluorophenyl)-6,8-dimethoxyisoquinoline (compound 3b).
[0106]
[0107] The synthetic method of embodiment 2 is the same as the synthetic method of above-mentioned embodiment 1.
[0108] Yield: 35%; Structural parameters: 1 H NMR (400MHz, CDCl 3 )δ9.46(s,1H),8.06(dd,J 1 =5.6Hz,J 2 =8.8Hz, 2H), 7.81(s, 1H), 7.16(t, J=8.8Hz, 2H), 6.66(d, J=2.0Hz, 1H), 6.48(d, J=2.0Hz, 1H), 3.99(s,3H),3.93(s,3H), such as figure 2 shown.
Embodiment 3
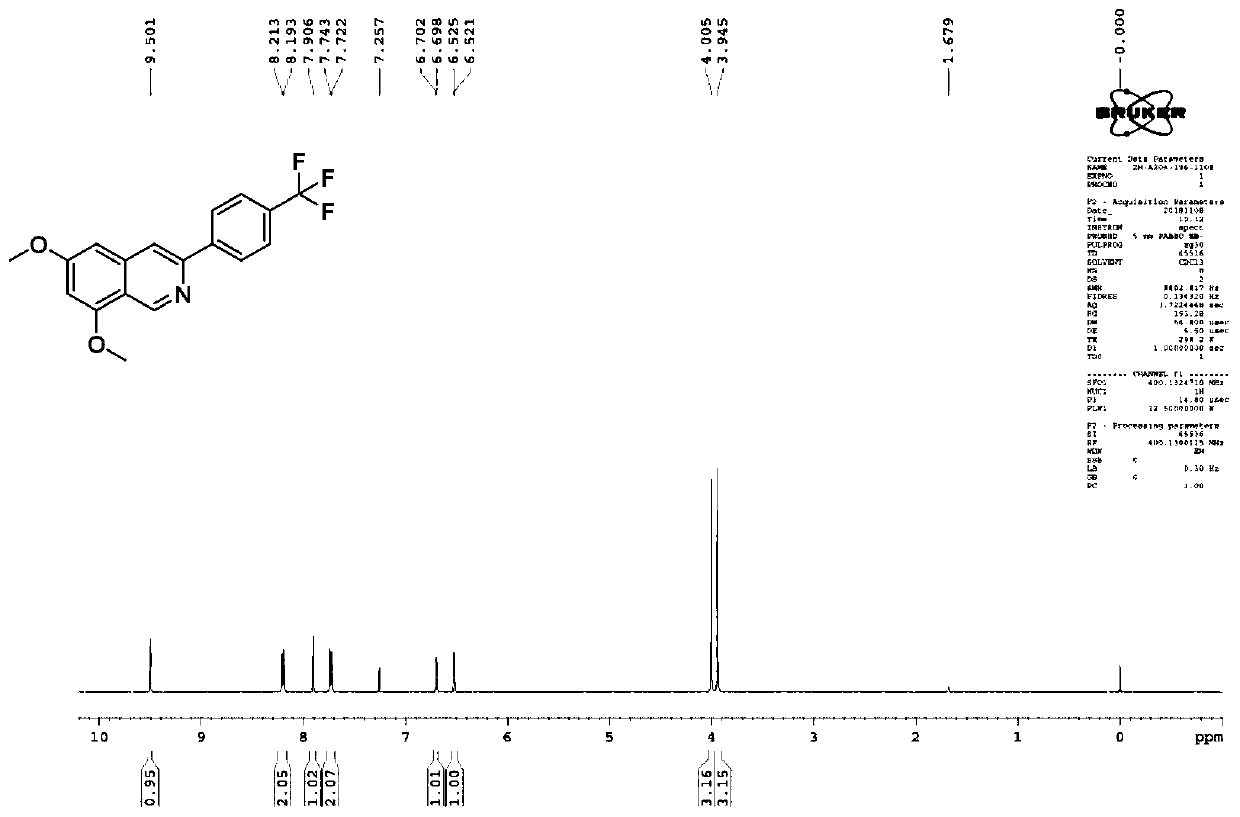
[0110] Synthesis of 6,8-dimethoxy-3-(4-(trifluoromethyl)phenyl)isoquinoline (compound 3c).
[0111]
[0112] The synthetic method of embodiment 3 is the same as the synthetic method of above-mentioned embodiment 1.
[0113] Yield: 35%; Structural parameters: 1 H NMR (400MHz, CDCl 3 )δ9.50(s,1H),8.20(d,J=8.0Hz,2H),7.90(s,1H),7.73(d,J=8.4Hz,2H),6.69(d,J=1.6Hz, 1H), 6.52(d, J=1.6Hz, 1H), 4.00(s, 3H), 3.94(s, 3H), such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com