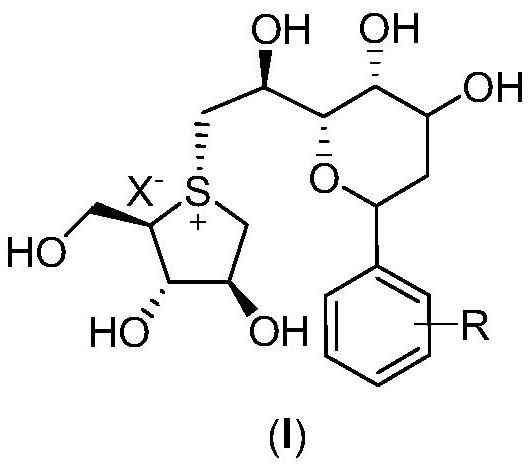
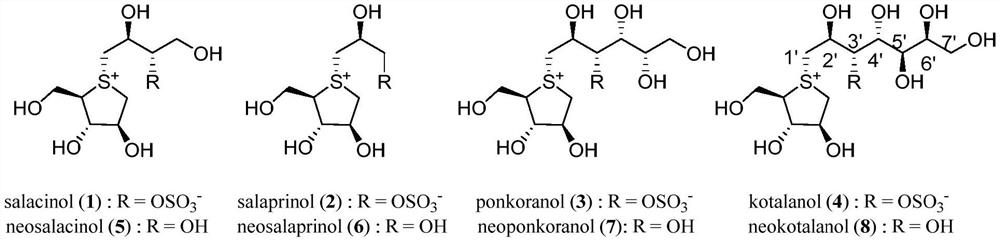
Sulfonium salt derivative as well as preparation method and medical application thereof
A derivative and sulfonium technology, applied in the field of chemical synthesis, can solve the problems of inability to reach the small intestine in form, and the compound is not acid-resistant, and achieve the effect of ingenious design, good tolerance, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
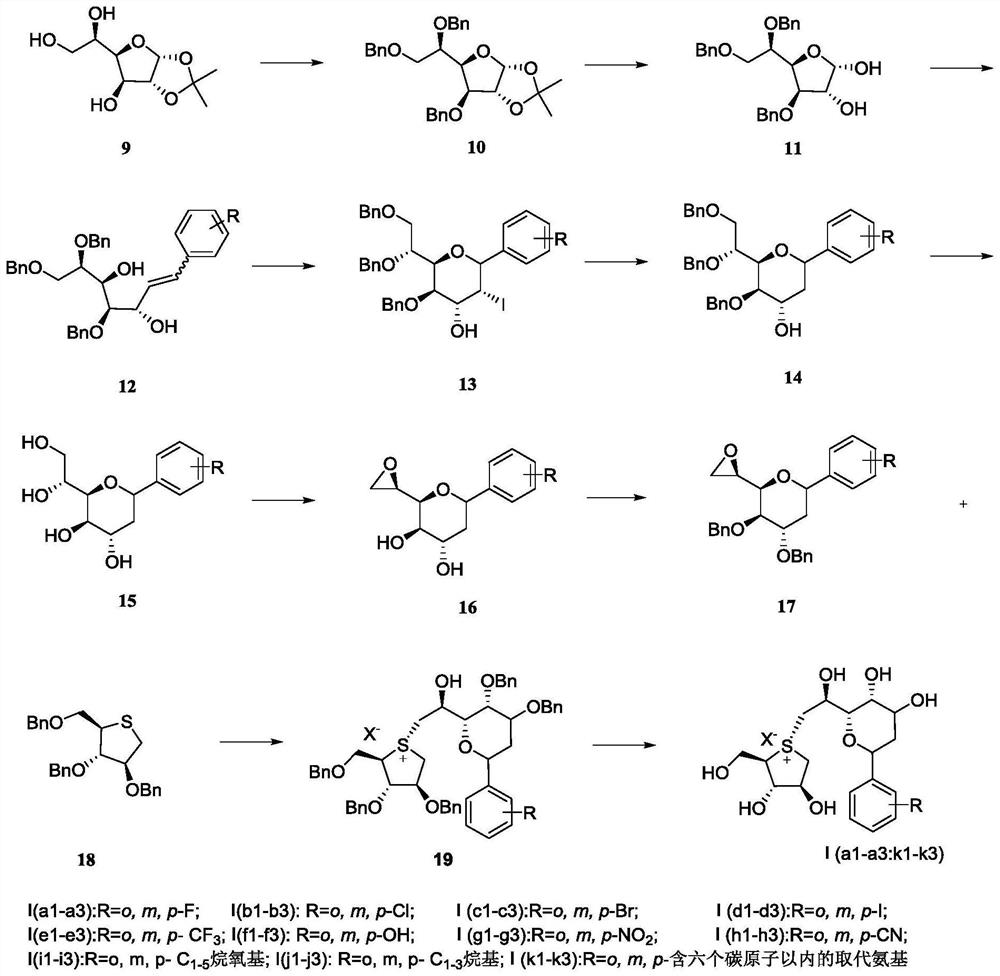
[0060] Preparation of 4,6,7-tri-O-benzyl-phenylheptenyl-3,5-diol
[0061]
[0062] Dissolve 1,2-O-isopropylidene-furan-D-glucose 9 (10g, 45.4mmol) in DMF (150mL), add NaH (3.6g, 149.9mmol) in batches under ice-water bath, and Stirring at low temperature for 30 min, BnBr (17.8 mL, 149.9 mmol) was added dropwise, and stirring was continued at room temperature for 2 hours after the addition was complete. After the completion of the reaction was monitored by a thin-layer silica gel plate, ice water was slowly added in an ice-water bath to quench. EA extraction, washing with water and saturated NaCl solution. Combined organic layers, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, make 100-200 mesh silica gel sand, separate and purify by chromatography column, PE:EA=50:1 and collect compound 10 (19.6 g, 40.4 mmol, 89%) as a yellow oil.
[0063] Compound 10 (12g, 24.5mmol), dissolved in 50mL of water, was stirred at room temperature and added to CF 3 COOH (50mL)...
Embodiment 2
[0067] Preparation of 2-I-4,6,7-tri-O-benzyl-1-C-phenylpyranoside
[0068]
[0069] Compound 12 (625 mg, 1.2 mmol) was dissolved in MeCN solution (10 mL), and NaHCO was added at -25°C 3 Powder (490mg, 3.6mmol), I 2 (910mg, 3.6mmol), continue to stir overnight at this temperature, after the completion of the TLC detection reaction, EA extracted 3 times, washed with water, washed with saturated NaCl, combined organic layers, anhydrous NaCl 2 SO 4 Drying, concentration under reduced pressure, 100-200 mesh silica gel sand, separation and purification by chromatography column, PE: EA = 15: 1 was collected to obtain compound 13 (511 mg, 0.8 mmol, 79%), a brown oily substance, namely 2-I- 4,6,7-Tri-O-benzyl-1-C-phenylpyranoside.
[0070] 1 H NMR (300MHz, CDCl 3)δ7.36–7.20(m,20H),4.80(d,J=10.4Hz,2H),4.72(dd,J=11.0,2.3Hz,1H),4.58–4.48(m,3H),4.46–4.40 (m,2H),4.24(d,J=9.1Hz,2H),3.95(dd,J=11.6,2.5Hz,2H),3.75(dd,J=10.7,2.0Hz,1H),3.59(dd, J=10.7,4.8Hz,1H). 13 CNMR (75MHz, CDCl 3...
Embodiment 3
[0072] Preparation of 2-deoxy-4,6,7-tri-O-benzyl-1-C-phenylpyranoside
[0073]
[0074] Compound 13 (378 mg, 0.6 mmol) was dissolved in THF (20 mL), added dry 10% Pd / C (30 mg), Et 3 N (100uL, 0.7mmol), oil bath 60°C in H 2 React in the environment for 3 hours, after the reaction is detected by TLC, filter out Pd / C with diatomaceous earth, spin dry the mother liquor, make sand with 100-200 mesh silica gel, separate and purify by chromatography column, PE:EA=10:1 and collect the compound 14 (300mg, 0.58mmol, 96%), yellow oily substance, namely 2-deoxy-4,6,7-tri-O-benzyl-1-C-phenylpyranoside.
[0075] 1 H NMR (300MHz, CDCl 3 )δ7.35–7.22(m, 20H), 4.82(t, J=10.6Hz, 2H), 4.59– 4.44(m, 5H), 4.23–4.00(m, 3H), 3.91(d, J=10.7Hz ,1H),3.73(dd,J=10.6,4.7Hz,1H), 3.65(s,1H),2.12(t,J=13.1Hz,1H),1.83(d,J=13.5Hz,1H). 13 C NMR (75MHz, CDCl 3 ) δ142.6, 138.8, 138.6, 134.0, 128.39, 128.36, 128.3, 128.2, 127.8, 127.63, 127.59, 127.5, 127.4, 127.2, 126.0, 76.6, 74.3, 73.5, 56.3, 73.3, 93.3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com