Small molecule peptides and their uses
A technology for small molecular polypeptides and uses, which is applied in the direction of peptides, medical preparations containing active ingredients, peptide/protein components, etc., to achieve the effect of simple synthesis method, easy structure, and good α-glucosidase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A small molecular polypeptide, the amino acid sequence of the small molecular polypeptide is shown in SEQ ID No.1;
[0017] SEQ ID No. 1: ICKKMMKKSTLLQDDIL.
[0018] The isoelectric point of the small molecule polypeptide is 9.7.
[0019] The molecular weight of the small molecule polypeptide is 2008.54g / mol.
[0020] The preparation method of the small molecule polypeptide:
[0021] Choose amino acid-King resin as the carrier (resin), fully swell the resin with dichloromethane, add the first Fmoc-protected amino acid, wash several times with dimethylformamide, and use appropriate concentration of DBLK to replace the Fmoc-protecting group Then, wash it several times with dimethylformamide, wash away DBLK, weigh the appropriate condensing agent benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate and activate reagent methylmorpholine and the second Fmoc-protected amino acid (Fmoc-Cys-OH) at the C-terminus (Fmoc-Cys-OH) for coupling, and detected by ninhydrin...
Embodiment 2
[0023] Determination of α-glucosidase activity of small molecule polypeptides in vitro.
[0024] All tests were carried out with a Microplate reader ELX808TM microplate reader (BioTek, USA) at 37°C. The data analysis software uses Origin software for data processing, and uses acarbose as a reference substance.
[0025] (1) Preparation of inhibitor stock solution: the tested inhibitors were formulated into 10 mM DMSO solution (the DMSO solution of the polypeptide of Example 1).
[0026] (2) Preparation of enzyme stock solution: α-glucosidase was purchased from Sigma Company of the United States; 1 mg / mL was prepared respectively with phosphate buffer solution of pH=6.8.
[0027] (3) Preparation of substrate stock solution: p-nitrophenyl glucoside (PNPG) was used as substrate, purchased from Sigma Company; 10 mg / mL was prepared respectively with phosphate buffer solution of pH=6.8.
[0028] (4) Preparation of stop solution: Sodium carbonate (Na2CO3) was purchased from Shanghai...
Embodiment 3
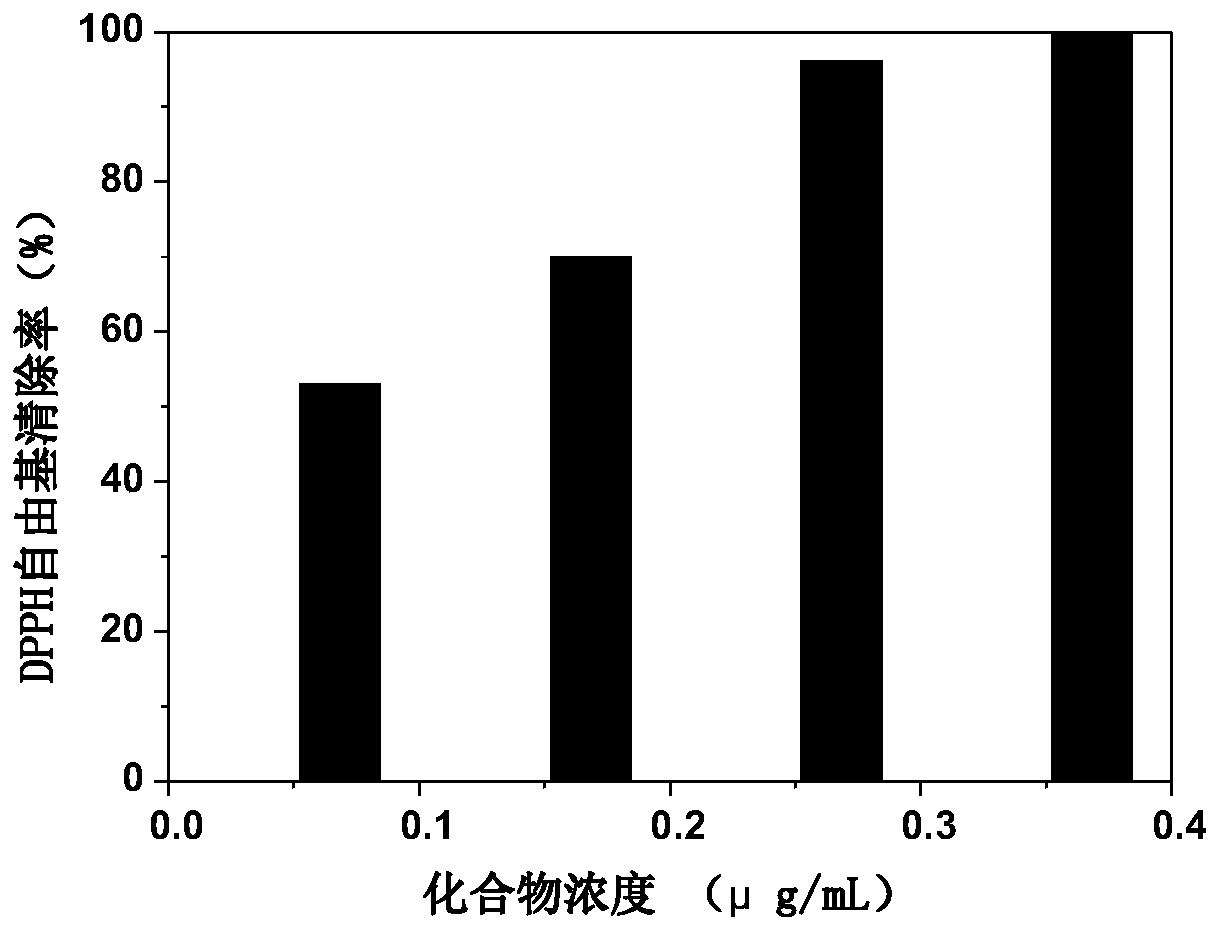
[0038] Take 0.1 ml of the DMSO solution (0.2, 0.4, 0.6, 0.8 μg / ml) of the polypeptide of Example 1 and add it to 8 ml of 0.004% 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) solution . Absorbance was measured at the maximum wavelength (517nm) until equilibrium. The formula for calculating the clearance rate is as follows:
[0039] S%=(1-A 样品 ) / A 空白 ×100%. Among them, A 空白 Absorbance of DPPH solution without adding peptide DMSO solution, A 样品 is the absorbance of the DPPH solution added to the DMSO solution of the compound. The result is as figure 1 shown. It can be seen that the polypeptide molecule of the present invention has excellent antioxidant performance, and the scavenging ability for DPPH free radicals can reach more than 99.5% at a relatively low concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com