Catalyst for ammonia synthesis reaction
A catalyst, a technology for synthesizing ammonia, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, preparation/separation of ammonia, etc., can solve the problems of unrealizable, obvious hydrogen poisoning effect of ruthenium-based catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Li 4 RuH 6 Sample preparation process: in an argon glove box, accurately weigh lithium hydride (LiH) 0.1197g, ruthenium powder (Ru) 0.3804g (molar ratio LiH:Ru=4:1), mix the two and place them in a self-made stainless steel ball mill in the can. After sealing the ball milling tank, put it into a planetary ball mill (Fischt PM400), the ball milling condition is 150rpm, and the ball milling time is 3 hours. In an argon glove box, the resulting sample was placed into a quartz liner, which was then placed in a stainless steel reactor, which was evacuated and flushed into 10barH 2 , and then heated up to 480 ° C for 12 hours, and after it was naturally cooled to room temperature, the obtained sample was put into a glove box for use.
[0019] Li 4 RuH 6 -BM sample preparation process: accurate weighing of Li 4 RuH 6 0.1000 g was placed in a self-made ball mill jar, and after the ball mill jar was closed, it was loaded into a planetary ball mill (Fischt PM400), the bal...
Embodiment 2
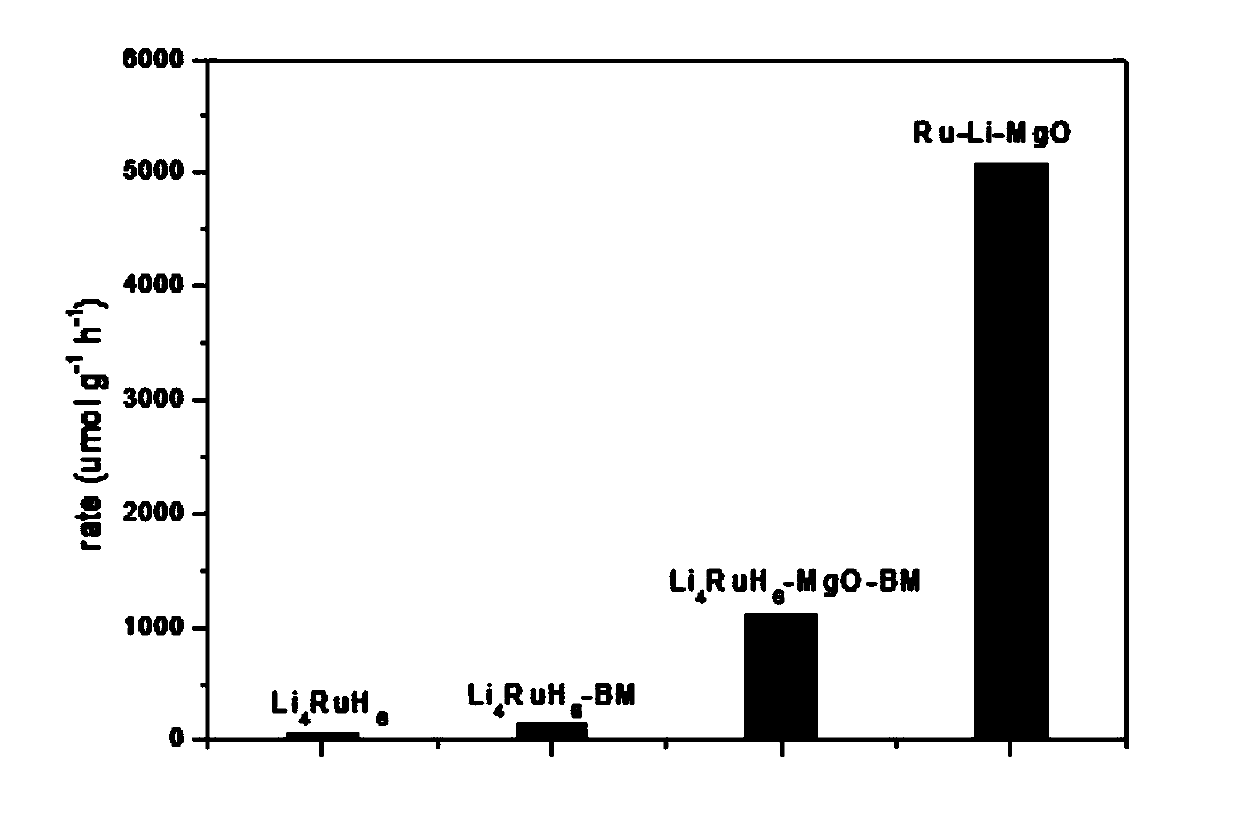
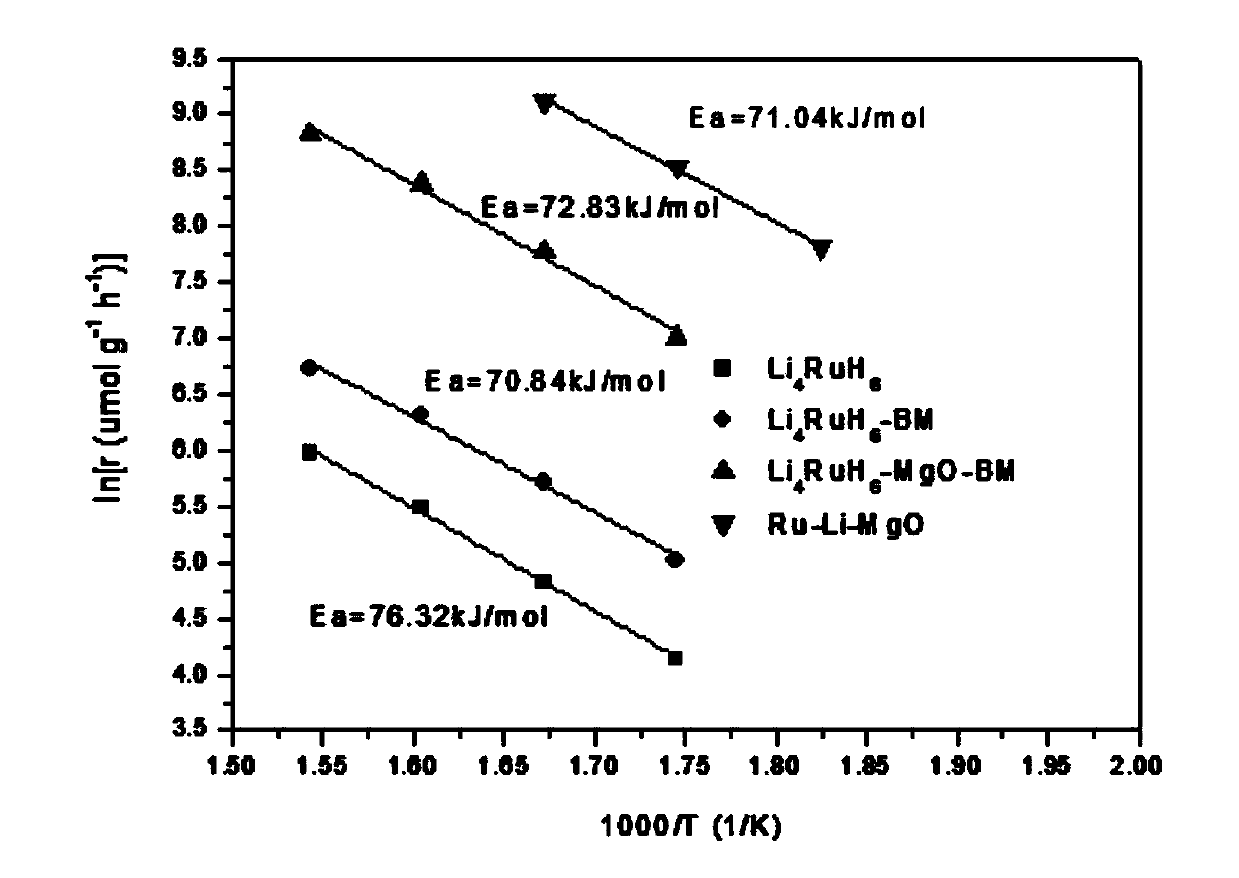
[0024] Test Li 4 RuH 6 , Li 4 RuH 6 -BM, Li 4 RuH 6 The activity of -MgO-BM and Ru-Li-MgO changes with temperature (reaction conditions: catalyst 30mg, flow velocity 30ml / min, pressure 1bar, N 2 :H 2 =3:1 (molar ratio)), the activation energy was calculated according to the Arrhenius equation. like figure 2 As shown, it can be seen that four kinds of Li with different dispersion 4 RuH 6 have similar activation energies, both close to 70kJ / mol, indicating that the Li 4 RuH 6 After dispersion, its active sites did not change.
Embodiment 3
[0026] Using the same method to prepare highly dispersed Ru-K-MgO (accurately weighed Ru-MgO 0.050g, K0.0077g), Ru-Ca-MgO (accurately weighed Ru-MgO 0.050g, Ca 0.0079g) and Ru-Na -MgO (accurately weigh 0.050g of Ru-MgO, 0.0043g of Na), Ru-Ba-MgO (accurately weigh 0.050g of Ru-MgO, 0.0272g of Ca).
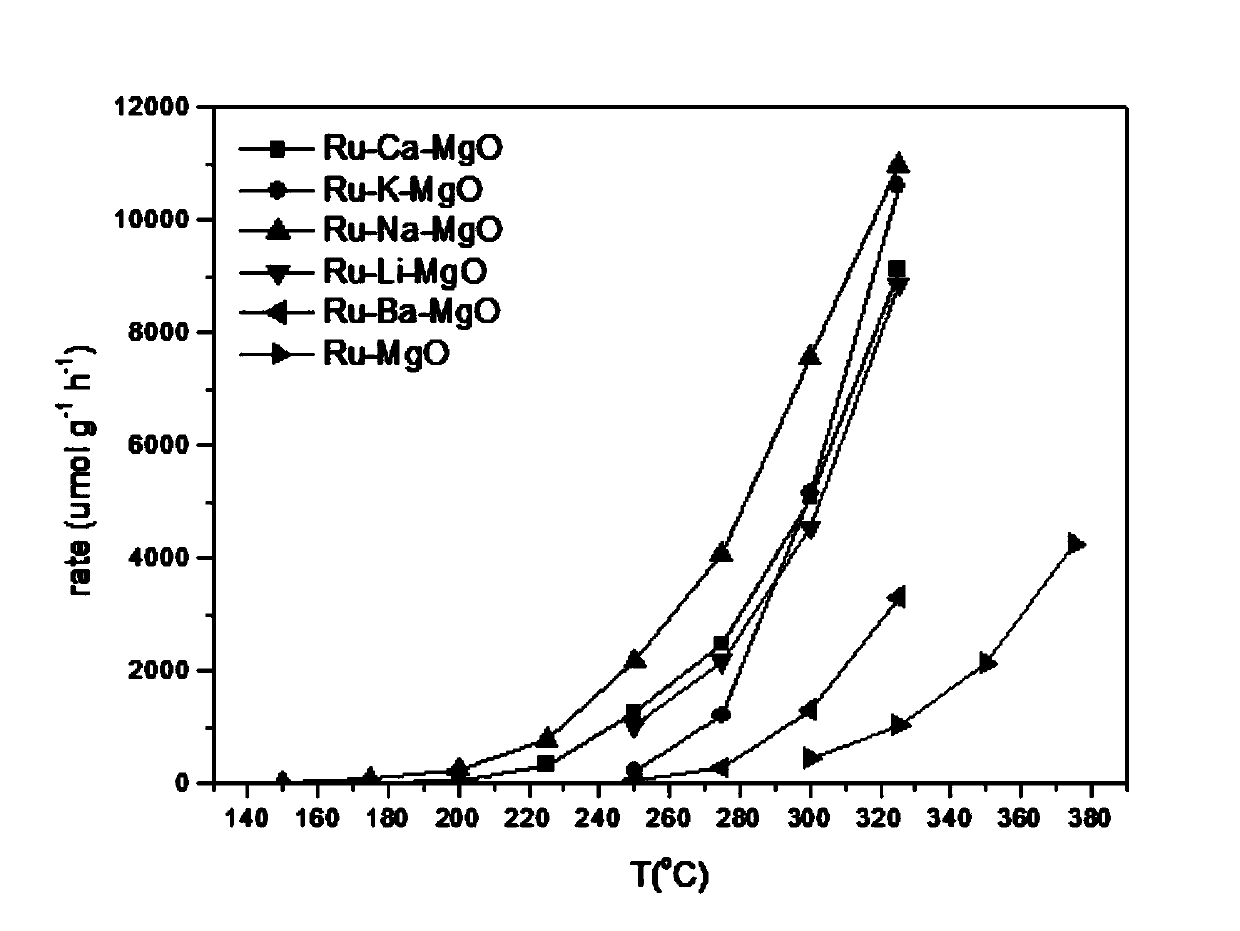
[0027] Accurately weigh 0.030g of Ru-Li-MgO, Ru-K-MgO, Ru-Ca-MgO-, Ru-Na-MgO, Ru-Ba-MgO and the reference catalyst Ru-MgO respectively, and place them in a fixed-bed stainless steel reaction device. Test its activity curve (reaction conditions: catalyst 30mg, flow velocity 30ml / min, pressure 1bar, N 2 :H 2 =3:1 (molar ratio)). like image 3 As shown, it can be seen that Ru-Li-MgO, Ru-MgO, Ru-Ca-MgO, Ru-Na-MgO, Ru-Ba-MgO all exhibit excellent catalytic activity. Among them, Ru-Na-MgO is active (24umol / g) at 150°C and 1bar cat / h), and the ammonia formation rate increases gradually with increasing temperature, which is higher than that of Ru-Li-MgO, Ru-K-MgO, Ru-Ca-MgO and Ru-Ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com