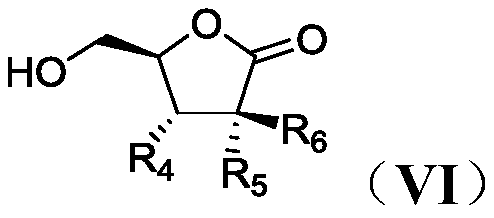
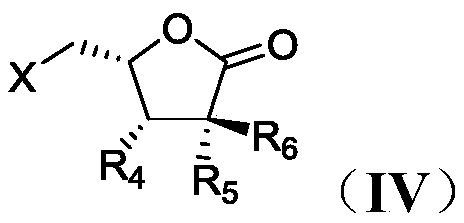
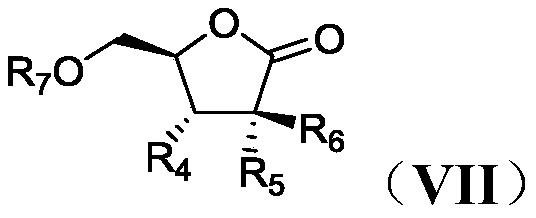
Process for preparation of lactone derivatives and intermediates thereof
一种化合物、独立地的技术,应用在制备内酯衍生物领域,能够解决产品de值不能达到所需水平等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Embodiment 1: Preparation of 2-fluoropropionyl chloride (3)
[0200]
[0201] Chlorosulfonic acid (660 mL, 10 mol, 20 eq) was added to a solution of phthaloyl dichloride (1.4 L, 10 mol, 20 eq) and ethyl 2-fluoropropionate (600 g, 5 mol) at room temperature . The solution was heated at 120°C for 4 hours. 2-(R)-Fluoropropionyl chloride was distilled from the reaction mixture under reduced pressure and recovered as a colorless oil (320 g, 58.2%). 1 H-NMR (CDCl 3 , 400MHz): δ5.08 (dq, J = 48.8, 6.8Hz, 1H), 1.63 (dd, J = 22.8, 6.8Hz, 3H).
Embodiment 2
[0202] Example 2: Preparation of (4R)-3-(2-fluoropropionyl)-4-isopropyloxazolidin-2-one (4)
[0203]
[0204] Under a nitrogen atmosphere at -50°C, n-butyllithium (2.5M in hexane, 30mL, 75mmol, 1.1eq) was added to 4-(R)-4-isopropyl-2-oxazolidinone ( 8.8g, 68.2mmol, 1 equivalent) in dry THF (80mL) solution. After 30 minutes, 2-fluoropropionyl chloride (6.8 mL, 0.9 equiv) was added, and the solution was stirred at -50°C for 4 hours. Then with saturated NH 4 Cl solution (50mL) quenched the reaction, extracted with MTBE (80mL*2), washed with brine and washed with MgSO 4 dry. The solvent was removed under reduced pressure. The product was purified by silica (hexane / EtOAc=10 / 1) and recovered as a brown oil (9 g, 74.8%). 1 H-NMR (CDCl 3 ,400MHz):δ6.00(dm,J=49.2Hz,1H),4.27-4.53(m,3H),2.43(dm,J=52.6Hz,1H),1.63(td,J=23.2Hz,3H) , 0.92 (dq, J=17.8Hz, 6H).
Embodiment 3
[0205] Example 3: Preparation of (4S)-3-(2-fluoropropionyl)-4-isopropyloxazolidin-2-one (5)
[0206]
[0207] Under a nitrogen atmosphere at -50°C, n-butyllithium (2.5M in hexane, 75mL, 187mmol, 1.1 equiv) was added to 4-(S)-4-isopropyl-2-oxazolidinone ( 22g, 170mmol, 1 equivalent) in dry THF (200mL) solution. After 30 minutes, 2-fluoropropionyl chloride (17 mL, 153 mmol, 0.9 equiv) was added, and the solution was stirred at -50°C for 1 hour. After the starting material is completely consumed, it is then washed with saturated NH 4 Cl solution (125mL) quenched the reaction, extracted with MTBE (200mL*2), washed with brine and washed with MgSO 4 dry. The solvent was removed under reduced pressure. The product was purified by silica (Hexane / EtOAc = 10 / 1) and recovered as a brown oil (34 g, 83.3%). 1 H-NMR (CDCl 3 ,400MHz):δ5.93(dm,J=48.8Hz,1H),4.19-4.17(m,3H),2.35(dm,J=52.8Hz,1H),1.55(td,J=23.6Hz,3H) , 0.85 (dq, J=18Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com