Preparation method of selective PPAR delta agonist GW501516
An agonist and selective technology, which is applied in the synthesis of pharmaceutical intermediates and the preparation of selective PPARδ agonist GW501516, which can solve the problems of synthetic safety hazards and achieve the effects of short steps, high reaction yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
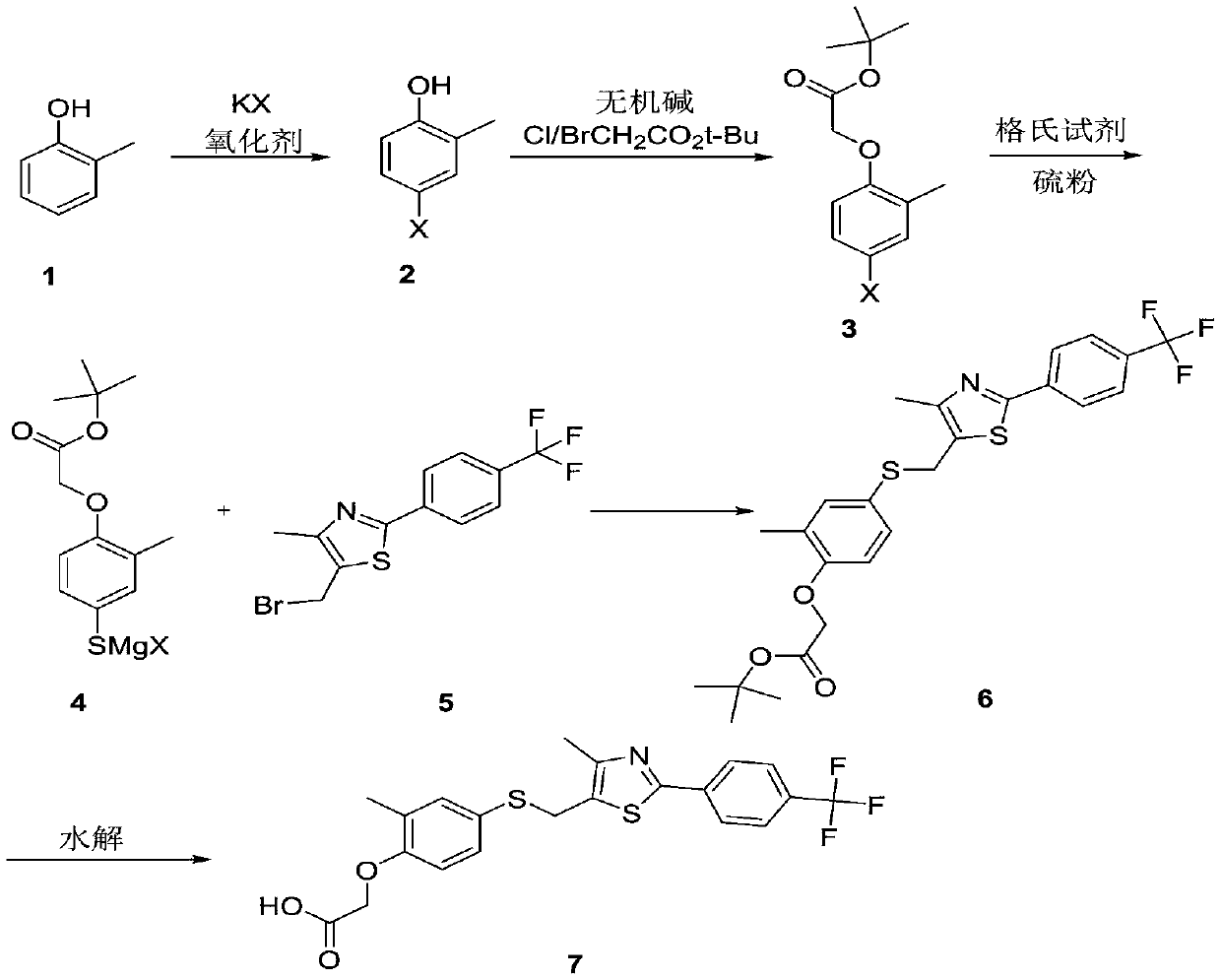
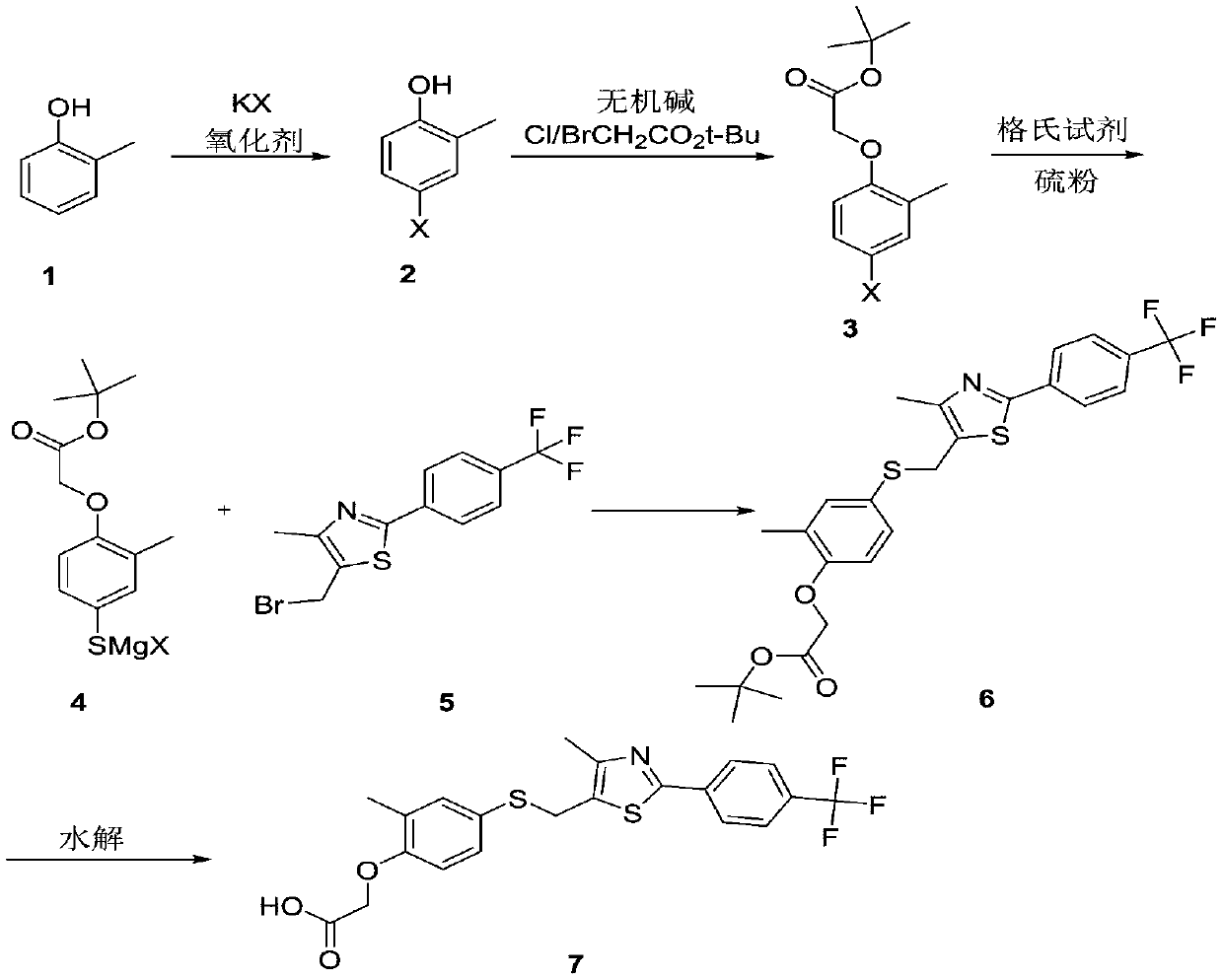
Embodiment 1
[0034]
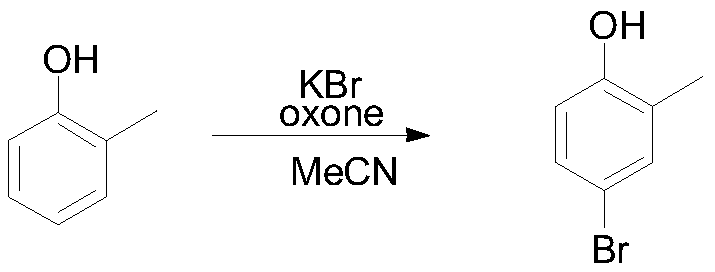
[0035] 108.1 g (1 mol) of o-cresol, 130.9 g (1.1 eq) of potassium bromide, and 2.5 Kg of acetonitrile were put into the reaction flask. Under stirring at room temperature, add 677g (1.1eq) of potassium monopersulfate compound salt in batches under temperature control at 25-30°C, react at room temperature for 6 hours, HPLC detects that the raw material is 1 HNMR (400MHz, CDCl3): 7.36-7.34(d, 1H), 6.71-6.75(d, 1H), 6.58-6.53(dd, 1H), 4.73(s, 1H), 2.34(s, 3H).
Embodiment 2
[0037]
[0038] 108.1 g (1 mol) of o-cresol, 183 g (1.1 eq) of potassium iodide, and 2 Kg of methanol were put into the reaction flask. Under stirring at room temperature, add 677g (1.1eq) of potassium monopersulfate compound salt in batches under temperature control at 25-30°C, react at room temperature for 8 hours, HPLC detects that the raw material is 1 HNMR (400MHz, CDCl3): 7.45-7.41(d,1H), 7.36-7.34(d,1H), 6.56-6.52(dd,1H), 4.78(s,1H), 2.20(s,3H).
Embodiment 3
[0040]
[0041] 108.1 g (1 mol) of o-cresol, 183 g (1.1 eq) of potassium iodide, and 0.6 Kg of acetonitrile were put into the reaction flask. Under stirring at room temperature, 340g (1.25eq) of 23% peracetic acid was added dropwise under temperature control at 25-35°C, reacted at room temperature for 5 hours, and the raw material was detected by HPLC 1 HNMR(400MHz, CDCl3):7.45-7.41(d,1H),
[0042] 7.36-7.34(d,1H),6.56-6.52(dd,1H),4.78(s,1H),2.20(s,3H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap