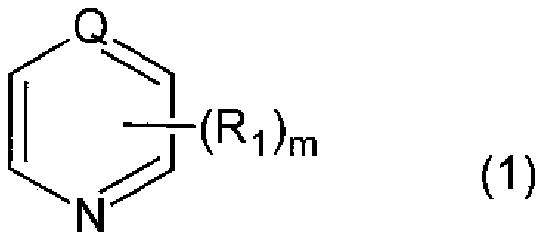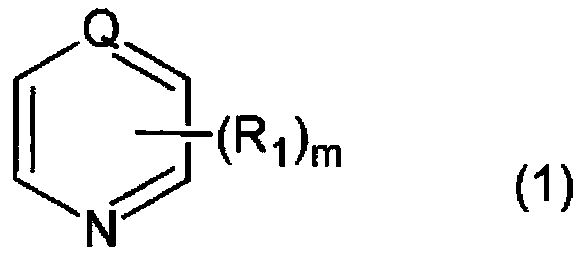Polymerizable composition for optical material, optical material, and production method for optical material
A technology for polymerizable compositions and optical materials, applied in the directions of optics, optical components, optical components, etc., to achieve the effects of excellent mold release, excellent manufacturing stability, and rib suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0228] Into the three-necked bottle of 2000ml, charge 2,5-bis(isocyanatomethyl)bicyclo-[2.2.1]-heptane, 2,6-bis(isocyanatomethyl)bicyclo-[2.2.1]- 54.3 parts by weight of heptane, 0.05 parts by weight of 2-(2-hydroxy-5-tert-octylphenyl)-2H-benzotriazole (BIOSORB 583), and 0.08 parts by weight of ZelecUN were made under a nitrogen atmosphere at 20°C. After completely dissolving, 0.06 parts by weight of β-picoline (pKa value=5.82) was added and stirred for 10 minutes until all of the mixture was dissolved. Next, 45.7 parts by weight of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane was added, moved to a bath at 10°C, stirred and mixed for 10 minutes, and then further heated at 0.20kPa Degassing was carried out under reduced pressure for 30 minutes to obtain a polymerizable composition.
[0229] A glass mold (upper mold) with 6 curved surfaces (upper mold) with a diameter of 78mm and a glass mold (lower mold) with 4 curved surfaces with a diameter of 78mm, and a lens with a cen...
Embodiment 2
[0231] In Example 1, except having made β-picoline into 2.50 weight part of 2-methylpyrazine (pKa value=1.50), the lens was obtained by the method similar to Example 1. Although slightly resin-colored, the lens was obtained. The evaluation results are shown in Table-1.
Embodiment 3
[0233] In Example 1, the lens was obtained by the method similar to Example 1 except having used 2.00 weight part of 3-chloropyridine (pKa value=2.84) as β-picoline. The evaluation results are shown in Table-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com