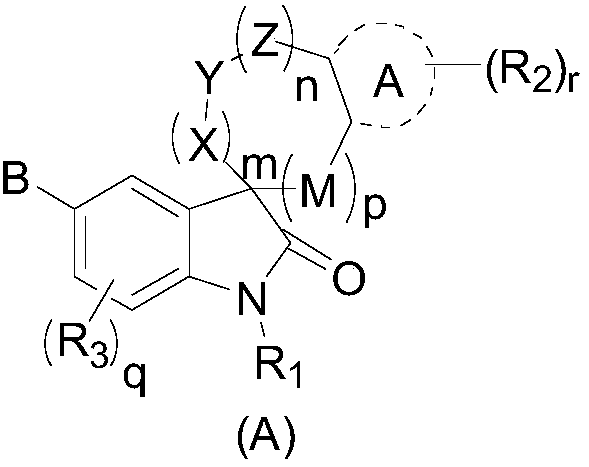
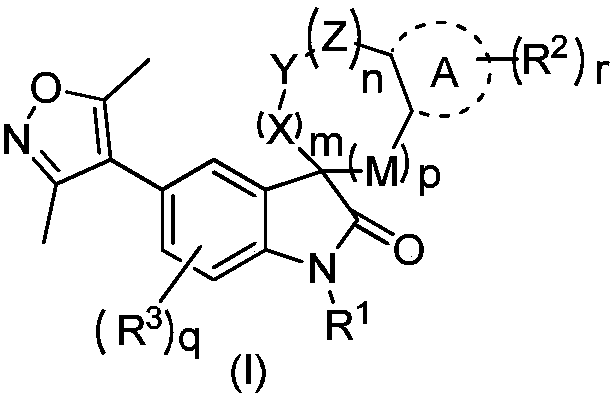
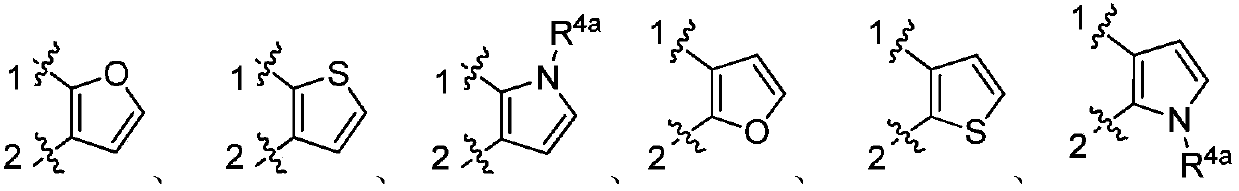
Indolone derivative as well as preparation method and application thereof
A technology of compounds and metabolites, applied in the field of medicine, can solve the problem of no BRD4 inhibitor on the market yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Example 1: 5-(3,5-dimethylisoxazol-4-yl)-3'-H-spiro[indole-3,1'-isobenzofuran]-2-one (compound 30 ) preparation
[0226]
[0227] The first step: preparation of compound 30b
[0228] Compound 30a (1.0g, 5.4mmol) was dissolved in 20mL MeOH, and NaBH was added under ice-cooling 4 (1.0 g, 5.6 mmol), reacted at room temperature for 2 h, the reaction solution was extracted with EA, concentrated, and purified by silica gel column chromatography to obtain compound 30b (1 g).
[0229] The second step: the preparation of compound 30e
[0230] Compound 30d (9.04g, 40mmol) was dissolved in 90mL DMF, NaH (2.4g, 60mmol) was added in batches at 0°C, stirred at room temperature for 30min, compound 30c was added dropwise, reacted at room temperature for 2h, and purified by silica gel column chromatography to obtain the compound 30e (7.2g).
[0231] The third step: the preparation of compound 30f
[0232] Compound 30b (0.54g, 2.9mmol) was dissolved in 10mL THF, n-BuLi (2.3mL, 5....
Embodiment 2
[0239] Example 2: Preparation of 5-(3,5-dimethylisoxazol-4-yl)spiro[indole-3,1'-isochroman]-2-one (compound 31)
[0240]
[0241] The first step: preparation of compound 31c
[0242] Compound 31b (1.28g, 6.36mmol) was dissolved in 10mL THF, n-BuLi (5.1mL, 12.7mmol) was added dropwise at -78°C, stirred at -78°C for 30min, and compound 31a (2.0g, 5.78 mmol) THF solution was added dropwise to the reaction system, reacted at -78°C for 30 min, then naturally raised to room temperature for 1 h, and was separated and purified by silica gel column chromatography to obtain compound 31c (1.5 g).
[0243] The second step: the preparation of compound 31d
[0244] Compound 31c, PPh 3 (1.26g, 4.81mmol) dissolved in 10mLTHF, N 2 Protected, DEAD (0.838g, 4.81mmol) was added dropwise at -10°C, reacted at room temperature for 1h, separated and purified by silica gel column chromatography to obtain compound 31d (1.3g).
[0245] The third step: the preparation of compound 31f
[0246] Com...
Embodiment 3
[0249] Example 3: 5'-(3,5-Dimethylisoxazol-4-yl)-2,2-dimethylspiro[[d][1,3]benzodioxin-4,3 Preparation of '-indoline]-2'-one (compound 32)
[0250]
[0251] The first step: preparation of compound 32c
[0252] Compound 32b (5.51 g, 32 mmol) was dissolved in 30 mL THF, N 2 protection, at -75°C, add n-BuLi dropwise to the system, after 1h, dissolve 32a (6.0g, 26mmol) in 50mL THF and add dropwise to the reaction system, naturally rise to room temperature and react overnight, pass through a silica gel column Chromatography afforded compound 32c (1.72).
[0253] The second step: the preparation of compound 32e
[0254] Compound 32c (1.72g, 5.41mmol), 32d (1.45g, 6.49mmol), K 2 CO 3 (1.86g, 13.52mmol) and Pd(dppf)Cl 2 (396mg, 0.54mmol) dissolved in 40mL dioxane and 8mL water, N 2 protected, reacted overnight at 100°C, and obtained compound 32e (500 mg) by silica gel column chromatography.
[0255] The third step: the preparation of compound 32
[0256] Compound 32e (172m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com