Preparation and application of optical switch spiropyran-peryleneimide compound
A technology of perylene imide and compound is applied in the preparation and application field of optical switch spiropyran-perylene imide compound, which can solve the problems of poor solubility, synthesis and application limitation, etc. The effect of large-scale industrial production and huge application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
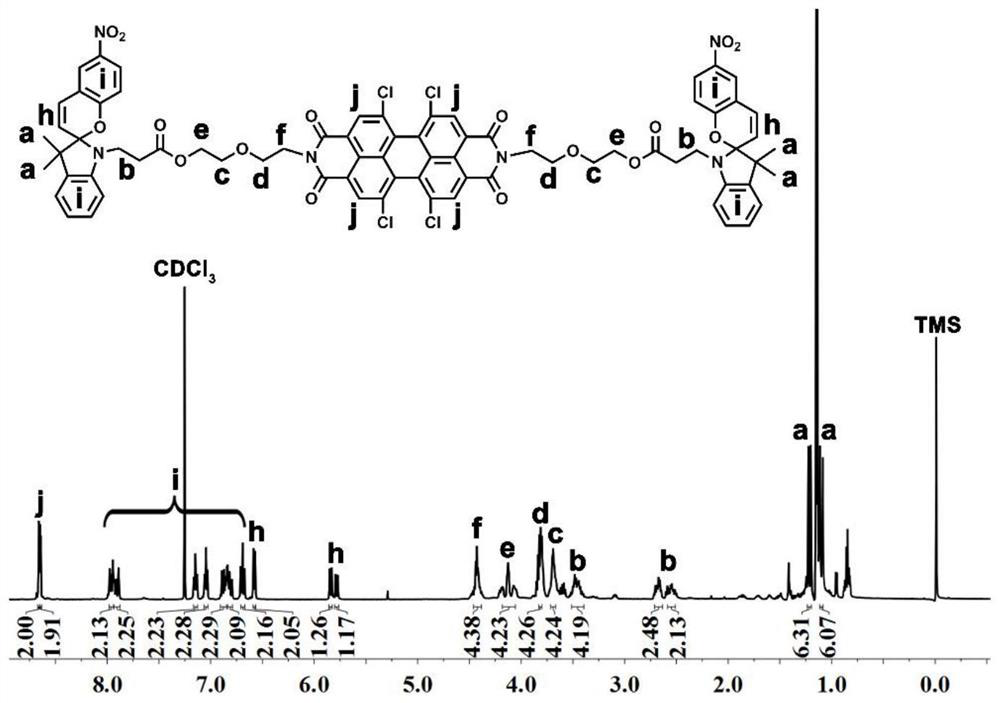
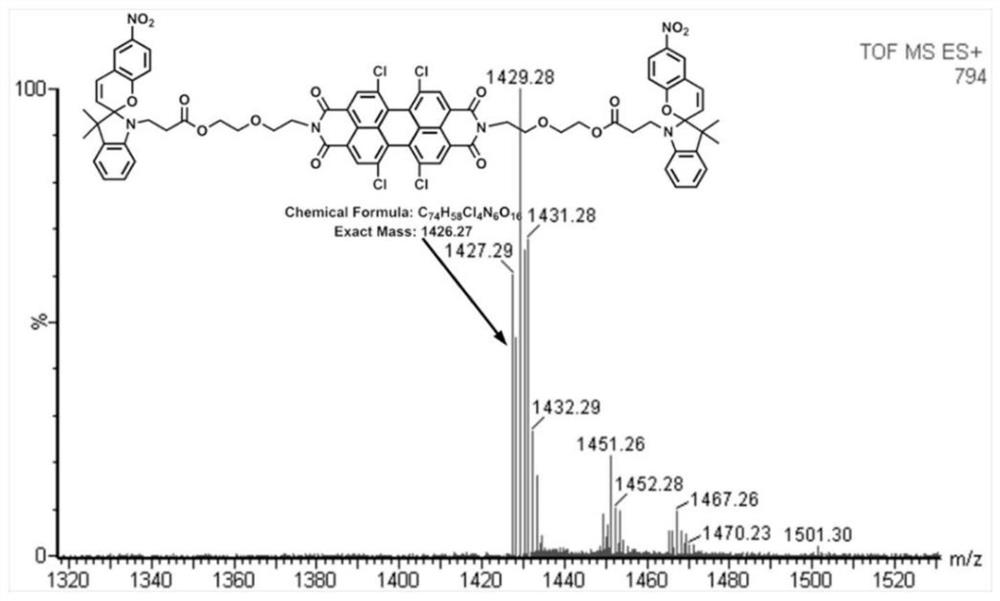
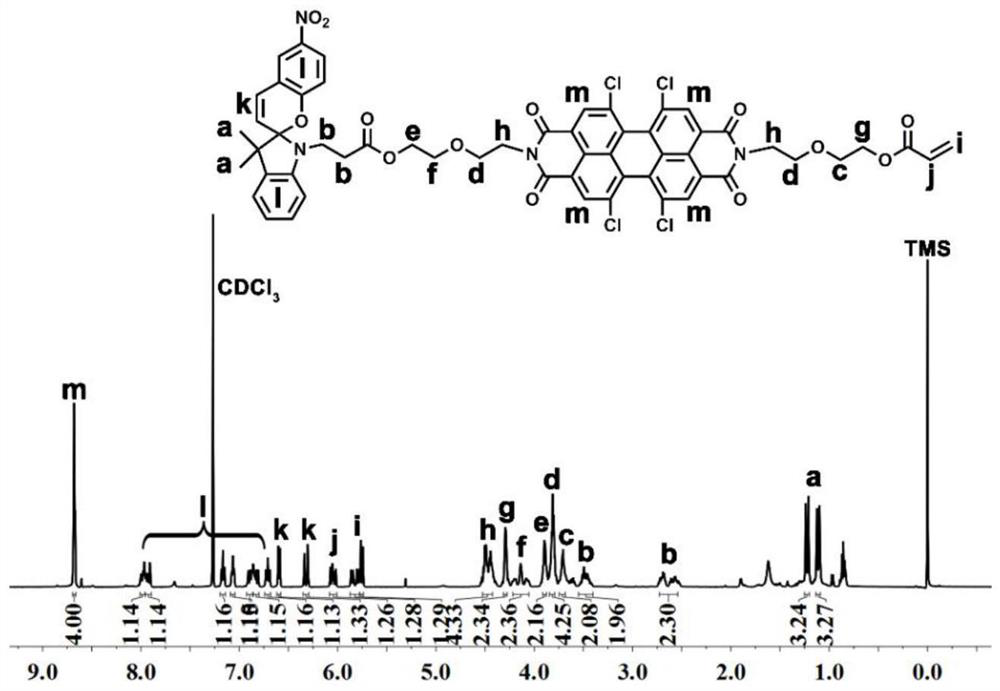
[0059] Embodiment 1: The preparation of a symmetrically substituted optical switch spiropyran-peryleneimide compound, the specific steps are as follows:
[0060] (1) Add 1,6,7,12-tetrachloro-3,4,9,10-perylenetetracarboxylic dianhydride (535 mg, 1.0 mmol) and diglycolamine (263 mg, 2.5 mmol) to Into a 25 mL two-neck round bottom flask, add 20 mL of dry pyridine and stir to dissolve, then reflux for 3 hours. After the reaction was completed, the solvent was concentrated, precipitated in petroleum ether, centrifuged, and dried in vacuo to obtain PDI-DGA (675 g, 96%) as a red solid.
[0061] (2) Dissolve the compound PDI-DGA (30 mg, 0.04 mmol) in 5 mL of anhydrous dichloromethane, and add N-carboxyethyl-3,3-dimethyl-6'- Nitroindoline spiropyran (40 mg, 0.08 mmol), N,N'-diisopropylcarbodiimide (9 μL, 0.06 mmol), and 4-dimethylaminopyridine (2.3 mg, 0.02 mmol) After the dropwise addition, turn to room temperature and stir the reaction for 24 hours. After the reaction was complete...
Embodiment 2
[0062] Embodiment 2: The preparation of a symmetrically substituted optical switch spiropyran-peryleneimide compound, the specific steps are as follows:
[0063] Dissolve the compound PDI-DGA (30 mg, 0.04 mmol) obtained in Example 1 (1) in 5 mL of anhydrous DCM, place at 0°C and add N-carboxyethyl-3,3-dimethyl -6'-nitroindoline spiropyran (80 mg, 0.16 mmol), N,N'-diisopropylcarbodiimide (12 μL, 0.08 mmol) and 4-dimethylaminopyridine (3.7 mg , 0.03 mmol), after the dropwise addition, turn to room temperature and stir for 24 hours. After the reaction was completed, the resulting mixture was concentrated and then purified by column using dichloromethane and anhydrous methanol (v:v = 100:1) as eluents to obtain a red solid SP-PDI (45 mg, 75%);
Embodiment 3
[0064] Embodiment 3: The preparation of a symmetrically substituted optical switch spiropyran-peryleneimide compound, the specific steps are as follows:
[0065] Dissolve the compound PDI-DGA (30 mg, 0.04 mmol) obtained in Example 1 (1) in 5 mL of anhydrous DCM, place at 0°C and add N-carboxyethyl-3,3-dimethyl -6'-nitroindoline spiropyran (100 mg, 0.2 mmol), N,N'-diisopropylcarbodiimide (14 μL, 0.1 mmol) and 4-dimethylaminopyridine (4.9 mg , 0.04 mmol), after the dropwise addition, turn to room temperature and stir for 24 hours. After the reaction was complete, the resulting mixture was concentrated and then purified by using dichloromethane and anhydrous methanol (v:v = 100:1) as eluents for column purification. After vacuum drying, a red solid SP-PDI (42 mg, 70%);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com