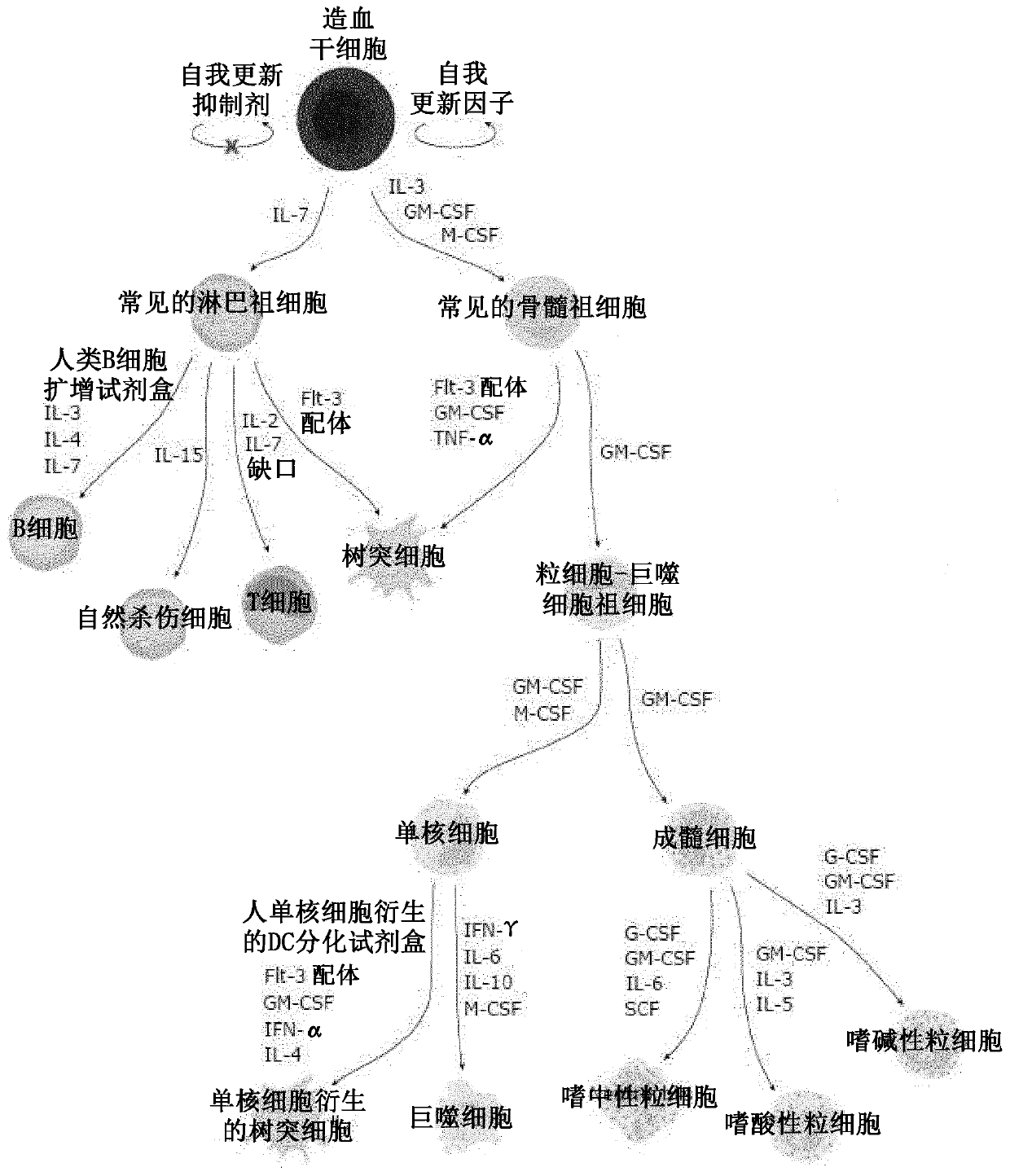
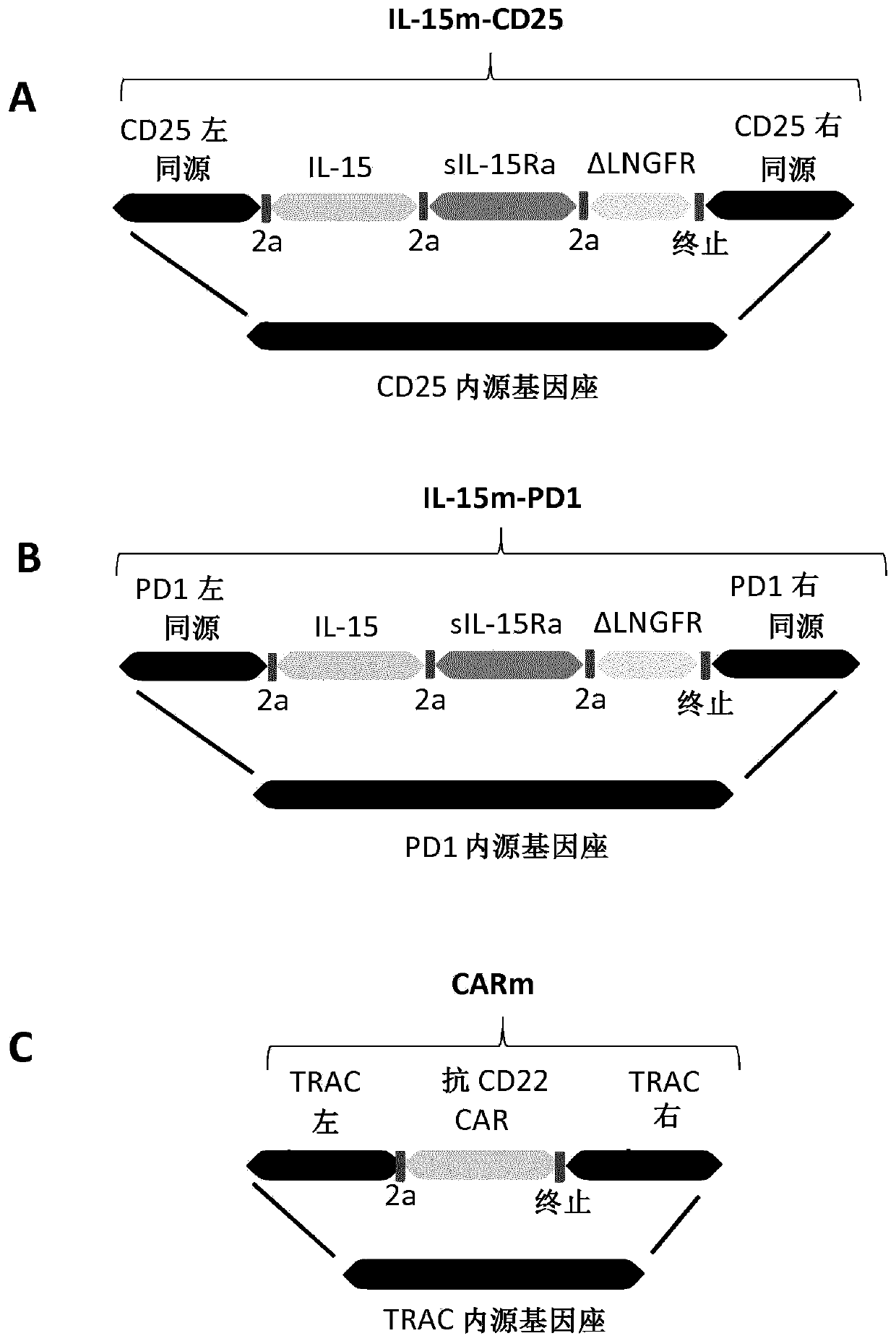
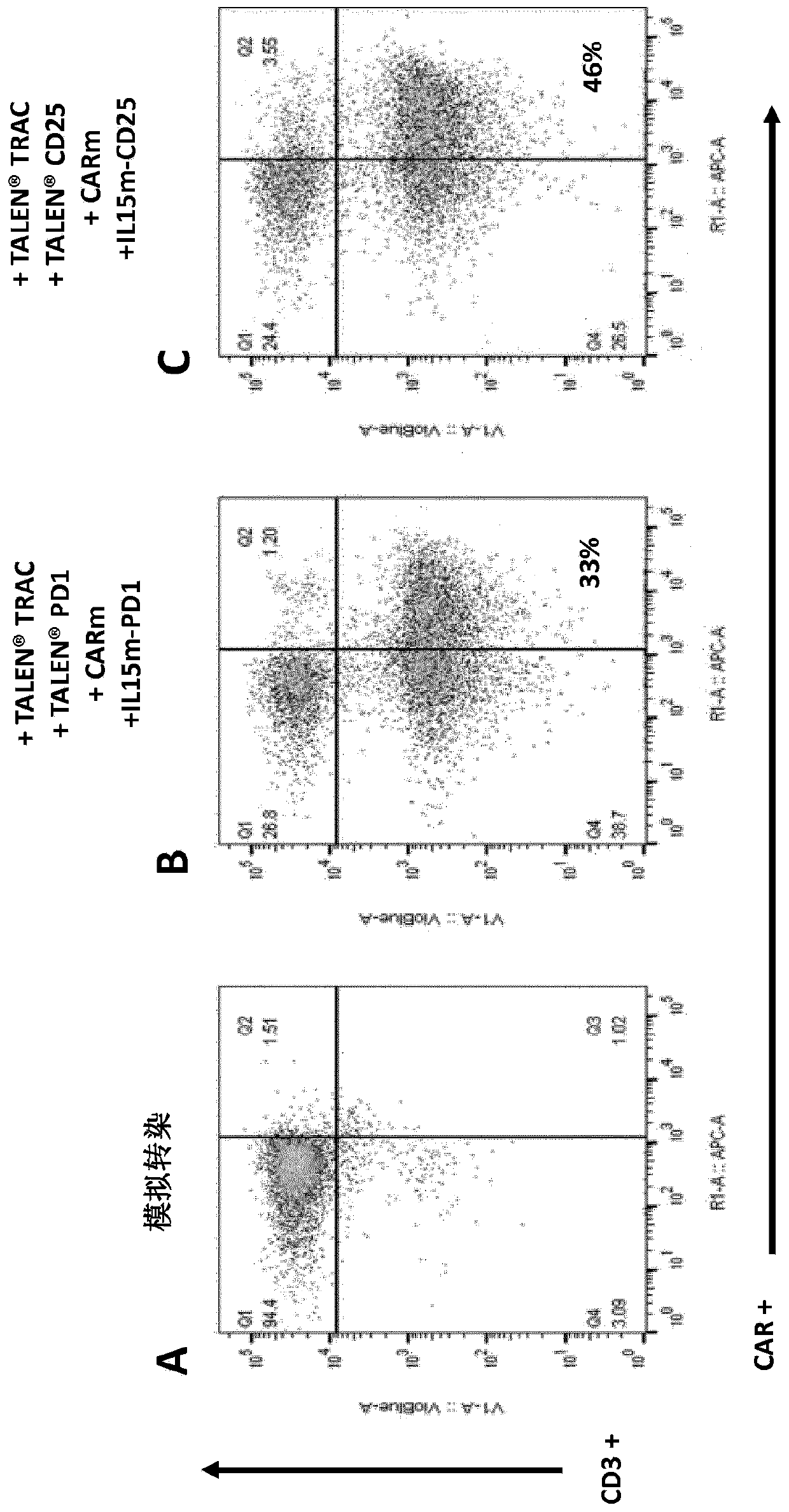
Targeted gene insertion for improved immune cells therapy
A technology of immune cells and therapy, applied in the field of sequence-specific endonuclease reagents and donor DNA vectors, which can solve the problems of reducing immune response and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0359] Example 1: AAV-driven homologous recombination at different loci in human primary T cells under the control of an endogenous promoter knocked out of an endogenous gene.
[0360] introduce
[0361] Sequence-specific endonuclease reagents such as (Cellectis, 8 rue de la Croix Jarry, 75013 PARIS) enables site-specific induction of double-strand breaks (DSBs) at desired loci in the genome. Repair of DSBs by cellular enzymes occurs mainly through two pathways: non-homologous end joining (NHEJ) and homology-mediated repair (HDR). HDR uses homologous DNA fragments (template DNA) to repair DSBs through recombination and can be used to introduce any gene sequence contained in the template DNA. As shown therein, recombinant adeno-associated virus (rAAV) with engineered nucleases such as The template DNA is delivered together to introduce site-specific DSBs.
[0362] Integrated Matrix Design
[0363] 1.1 Insertion of an apoptotic CAR at an upregulated locus knocked out of t...
Embodiment 2
[0382] Example 2: In T cells Mediated dual-targeted integration of IL-15 and CAR-encoded substrates
[0383] Material
[0384] X-vivo-15 was obtained from Lonza (cat#BE04-418Q), IL-2 was obtained from Miltenyi Biotech (cat#130-097-748), human serum AB was obtained from Seralab (cat#GEM-100-318), human T activator CD3 / CD28 was obtained from LifeTechnology (cat#11132D), QBEND10-APC was obtained from R&D Systems (cat#FAB7227A), vioblue-labeled anti-CD3, PE-labeled anti-LNGFR, APC-labeled anti- CD25 and PE-labeled anti-PD1 were obtained from Miltenyi (cat#130-094-363, 130-112-790, 130-109-021 and 130-104-892, respectively), 48-well treated plates (CytoOne, cat#CC7682- 7548), human IL-15 Quantikine ELISA Kit was obtained from R&D systems (cat#S1500), and ONE-Glo was obtained from Promega (cat#E6110). AAV6 batches containing different matrices were obtained from Virovek, PBMC cells were obtained from Allcells (cat#PB004F) and Raji-fluorescence was obtained after transduction of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com