A kind of bovine rotavirus, bovine coronavirus double inactivated vaccine and preparation method thereof
A dual inactivated vaccine and bovine rotavirus technology, applied in biochemical equipment and methods, viruses, vaccines, etc., can solve problems such as the difficulty of virus separation, achieve safe and reliable vaccines, and prevent calves from diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1---the preparation of bovine rotavirus, bovine coronavirus double inactivated vaccine (JY strain+YQ strain)
[0065] 1. Preparation of BRV-JY strain and BCV-YQ strain antigen by using roller bottle adherence
[0066] (1) Cultured cells: The seed cells MA-104 and HCT-8 were respectively recovered into cell flasks, grown for about 48 hours, digested with EDTA-trypsin cell dispersion, and passaged at a volume ratio of 1:4. Add serum-free DMEM medium (pH value is 7.0) for spinner flask culture, and when the cells are 90% to 100% full, they are used for continued passage or virus inoculation.
[0067] (2) Preparation of antigen (virus solution) for seedling preparation: Take the well-grown MA-104 cells, wash 2-3 times with PBS, and inoculate the BRV-JY strain at a dose of (V / V) 0.5%. inoculation. Serum-free DMEM medium (pH 7.0) was added for culture. After 5 to 7 days, when 80% of the cells appeared CPE, the spinner flask was placed at -20°C for 2 to 3 times o...
Embodiment 2
[0084] Embodiment 2---finished product inspection of bovine rotavirus, bovine coronavirus double inactivated vaccine (JY strain+YQ strain)
[0085] 1. Sterility test: According to the appendix of the current "Chinese Veterinary Pharmacopoeia", it should meet the regulations. The above test results are shown in Table 2.
[0086] Table 2 Physical properties, sterility test results
[0087]
[0088] 2. Safety inspection:
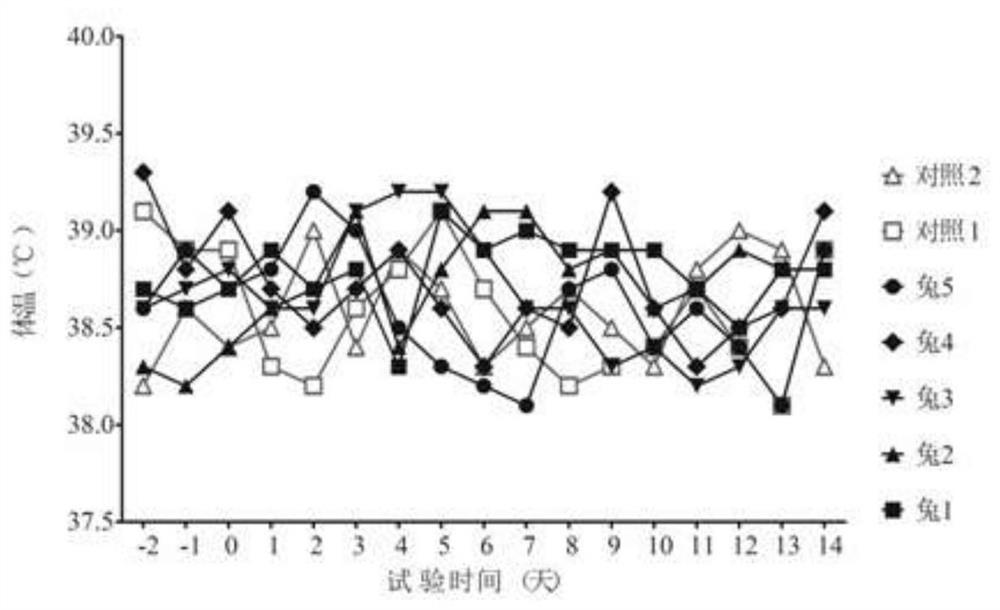
[0089] (1) Alternative animal test: 7 healthy rabbits of 1.5-2.0 kg were selected for the test, including 5 in the immunization group and 2 in the control group. The rabbits in the immunization group were injected subcutaneously with 1.0ml of vaccine (two-point injection, 0.5ml) / point), the control group rabbits were subcutaneously injected with 1.0ml of cell culture in each neck (two-point injection, 0.5ml / point), observed continuously for 7 days and measured the body temperature every afternoon at a fixed point. There is no abnormality to touch, no sym...
Embodiment 3
[0105] Example 3 - Antibody tracking test of newborn calves
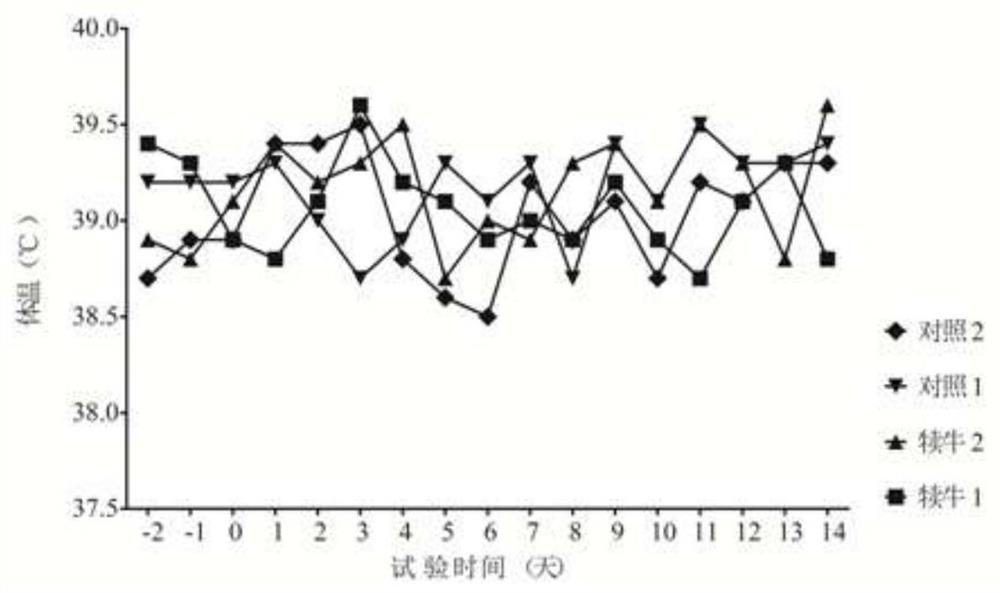
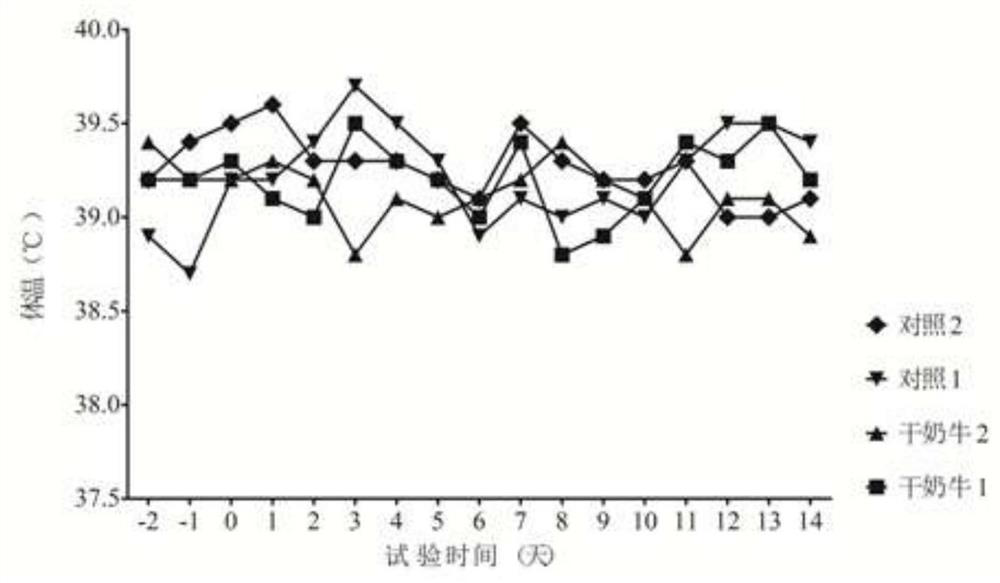
[0106] Select 10 dry cows with BRV, BCV antigen and antibody double negative (antigen negative means RT-PCR detection is negative, antibody negative means serum neutralizing antibody titer ≤ 1:4), including 5 in the immunization group and 5 in the control group , 2.0ml of vaccine was injected into each neck muscle of cows in the immunization group, and 2.0ml of cell culture was injected into each neck muscle of cows in the control group. After 21 days, the immunization was boosted in the same way. After the test cows gave birth, blood was collected from the newborn calves that did not eat colostrum, and the neutralizing antibody titers of BRV and BCV were detected. Blood was collected from calves 60 days after birth to detect BRV and BCV neutralizing antibody titers. The 4 / 5BRV neutralizing antibody titers of immunized cows calving ≥1:64, and the 4 / 5BCV neutralizing antibody titers ≥1:64, the results are shown in T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com