Human umbilical cord mesenchymal stem cell-derived stem cell factor microvesicle preparation and preparation method
A stem cell factor and stem cell technology, which is applied in the field of preparation and preparation of human umbilical cord mesenchymal stem cell-derived stem cell factor microvesicles, can solve the problem of limited total amount of microvesicles, influence of product uniformity, and influence on the preparation of microvesicles on cell state. and other problems, to avoid medical ethics problems, to achieve good product uniformity, and to facilitate large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
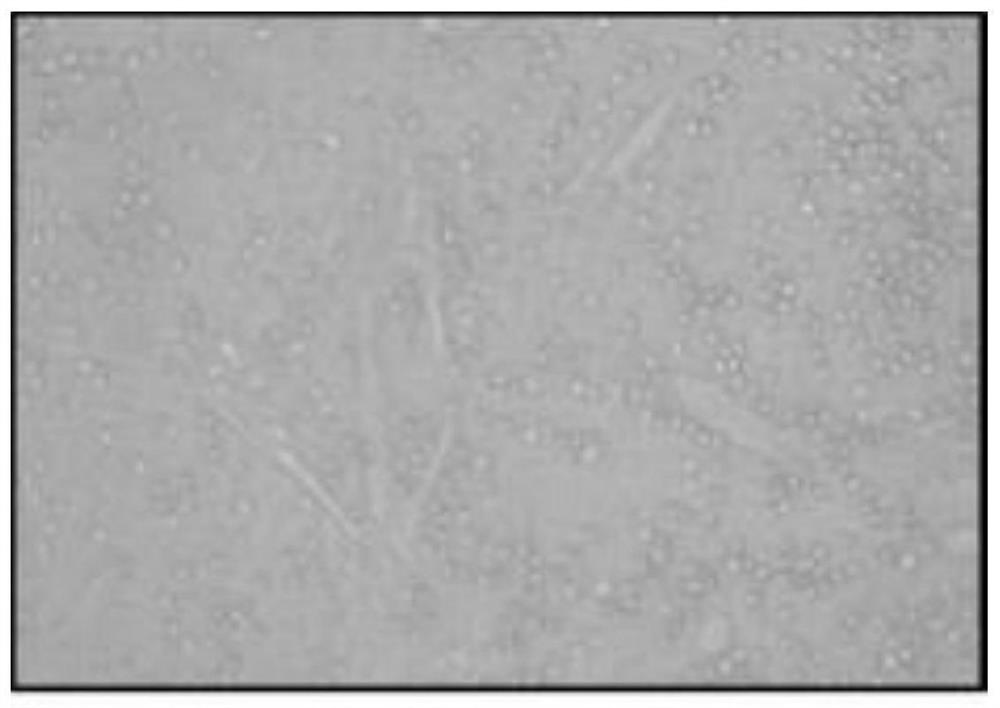
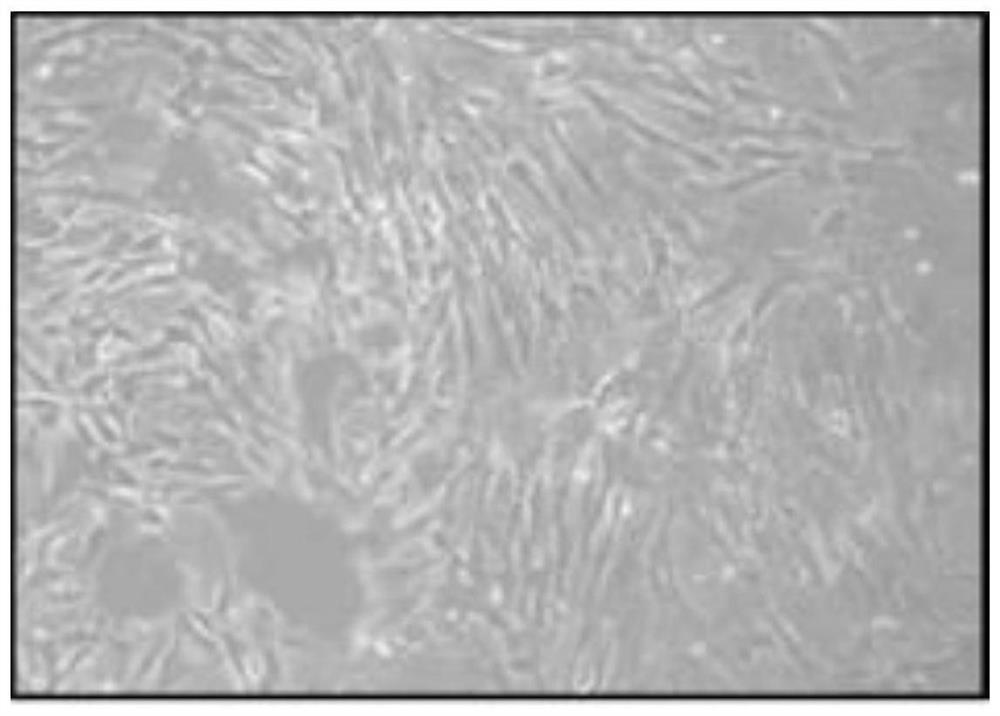
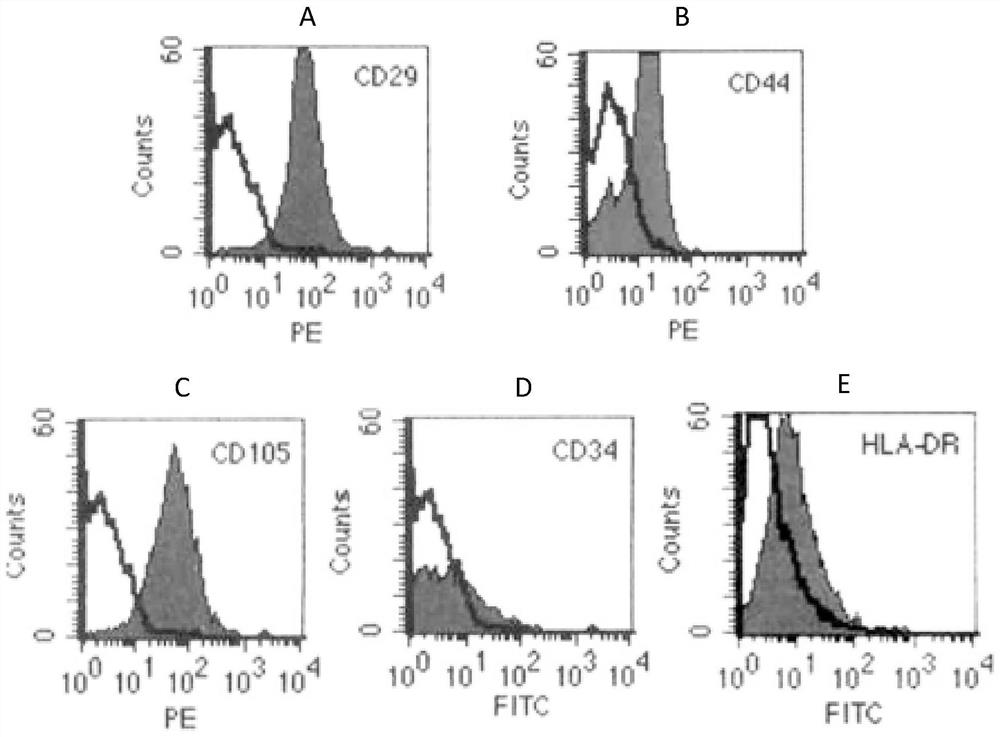
[0053] 1. Obtain primary human umbilical cord mesenchymal stem cells
[0054] (1) Aseptically obtain the umbilical cord of a full-term infant, place it in a sterile bag filled with sterile injection-grade normal saline at 4°C, in which the sterile injection-grade normal saline contains 50 μg / ml gentamicin, 2.5 μg / ml ml amphotericin B, 100 U / ml penicillin and 100 μg / ml streptomycin. Store the aseptic bag in a sterile storage cylinder at 4°C, and quickly transport it to a million-level cell laboratory for separation and preparation;
[0055] (2) Put the umbilical cord in a petri dish and place it in the cell operation table, remove 5cm lengths at both ends of the umbilical cord with sterile surgical instruments, wash the residual blood in the umbilical cord tissue with 4°C normal saline, and then place the umbilical cord at 4°C for medical use. Sterile injection-grade normal saline was cut longitudinally, the umbilical vessels were removed, and the amniotic tissue was completel...
Embodiment 2
[0075] Embodiment 2: Stability test of freeze-dried powder
[0076] Dissolve the lyophilized powder after being stored in a refrigerator at 4°C for half a year with sterile physiological saline at a concentration of 0.22mg / mL, and the total amount of sterile saline is 1ml; at the same time, the prepared lyophilized powder is immediately dissolved in sterile physiological saline The solution formed by saline (concentration is 0.25mg / mL, total amount is 1ml) was used as a control, and the expression levels of three representative human umbilical cord mesenchymal stem cell factors were detected by ELISA, as shown in Table 1.
[0077] Table 1 The concentration of representative cytokines VEGF, GEF, and FGF secreted by human umbilical cord mesenchymal stem cells detected by ELISA
[0078]
[0079] Note: In this embodiment, sterile physiological saline was used as the solvent each time the freeze-dried powder was dissolved to prepare the freeze-dried powder detection reagent, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com