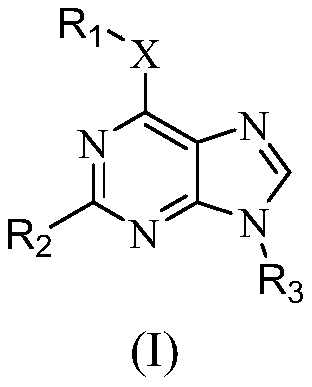
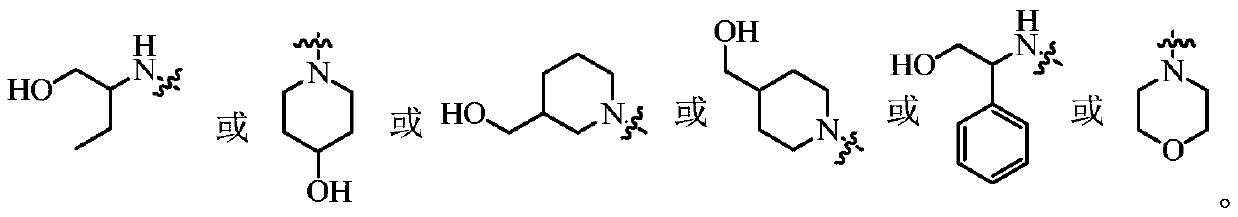
Purine compounds as Trk kinase inhibitors
A compound, purine technology, applied in the field of purine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] The preparation of embodiment 1 intermediate:
[0097] Preparation of 2-chloro-N-(4-fluorobenzyl)-9H-purin-6-amine:
[0098] The structural formula is as follows:
[0099] Add n-butanol (50mL) and 2,4-dichloropyrimidine (5g, 26.7mmol) into an eggplant-shaped bottle (250mL), stir to dissolve, then slowly add p-fluorobenzylamine (3.4g, 27mmol) and triethyl Amine (3.4g, 33.6mmol) was heated to 110°C for 3h. It was detected by TLC that the reaction was complete, cooled to 20° C., a solid precipitated out, filtered with suction, washed with n-butanol (5 mL), and dried to obtain a white solid product (5.5 g, yield 75%).
[0100] Preparation of 2-chloro-N-(4-fluorobenzyl)-9-isopropyl-9H-purin-6-amine (intermediate A1):
[0101] The structural formula is as follows:
[0102] Add DMSO (10mL) and 2-chloro-N-(4-fluorobenzyl)-9H-purin-6-amine (2g, 7.2mmol) into an eggplant-shaped flask (250mL), stir to dissolve, and store at 18-20°C Potassium carbonate (3.5 g, 25.3 mmol) a...
Embodiment 2
[0121] Example 2 Preparation of (S)-2-((6-(4-fluorobenzoyl)-9-isopropyl-9H-purin-2-ol) butanone-1-ol
[0122]
[0123] Add intermediate A1 (1.2g, 3.8mmol) and (R)-2-amino-1-butanol (3mL) into an eggplant-shaped flask (25mL), under nitrogen protection, heat to 160°C for 8h, and TLC detects that the reaction is complete , cooled to room temperature, added water (20 mL), extracted with ethyl acetate (20 mL×4), washed the organic layer with 50°C warm water (30 mL×2), dried over sodium sulfate, and evaporated most of the solvent under reduced pressure. Ethyl acetate (10 mL) was added to the residual liquid, crystals were precipitated, and white crystals (1 g, 70%) were obtained by filtration.
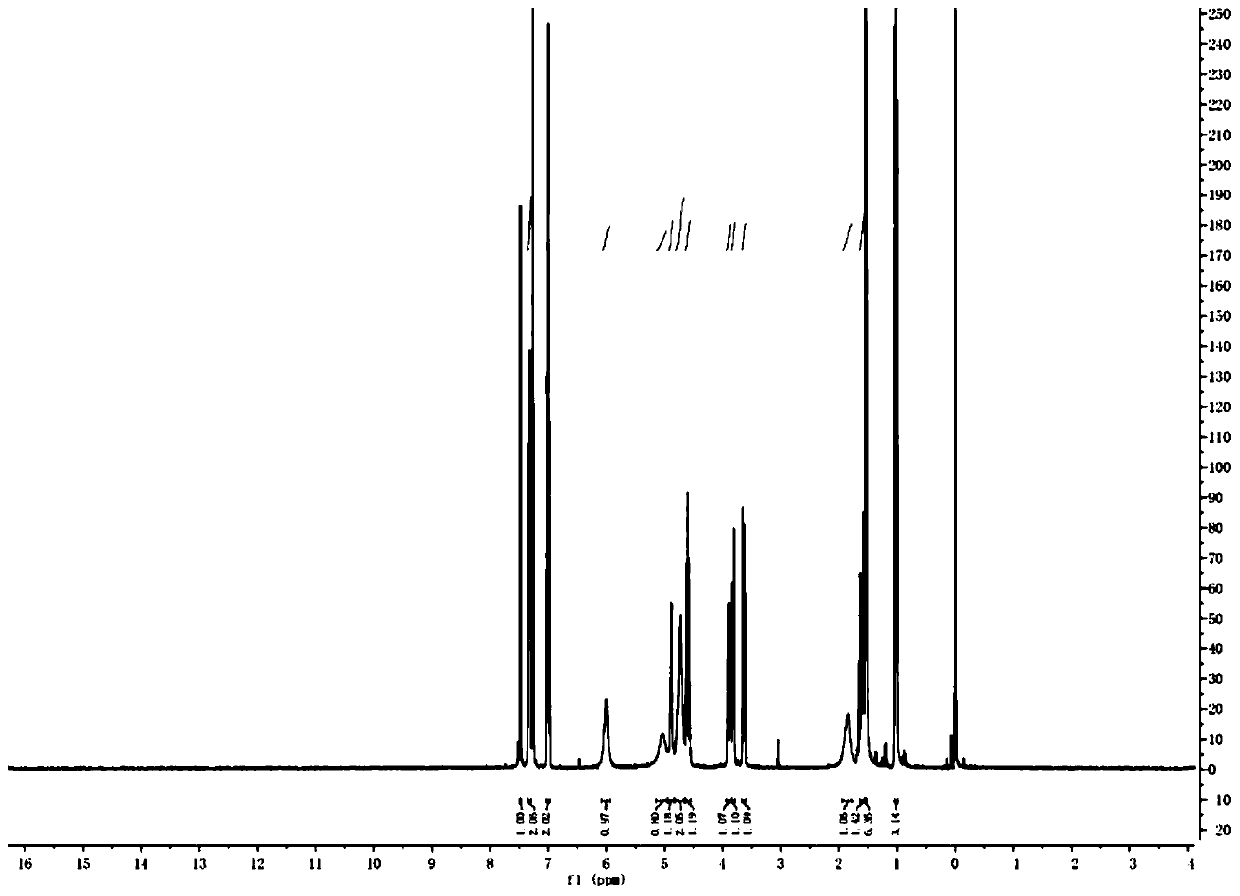
[0124] 1 H-NMR (400MHz, CDCl 3 )δ: 7.48(d,J=2.0Hz,1H),7.36–7.29(m,2H),7.04–6.97(m,2H),6.01(s,1H),5.04(s,1H),4.89(d ,J=6.1Hz,1H),4.73(s,2H),4.60(p,J=6.8Hz,1H),3.85-3.93(m,1H),3.82(dd,J=10.7,2.6Hz,1H) ,3.63(dd,J=10.7,7.7Hz,1H),1.85(s,1H),1.64–1.57(m,1H),1.53(d,J=6.7Hz,6H),0.97-0.08(m,3H...
Embodiment 3
[0125] Example 3 Preparation of 2-((6-(4-fluorophenyl)amino)-9-isopropyl-9H-purin-2-yl)amino)-2-phenethyl 1-ol
[0126] Synthesis was carried out in the same manner as that of Compound 1 except that DL-phenylglycinol was used instead of (R)-2-amino-1-butanol. The structural formula is as follows:
[0127]
[0128] 1 H-NMR (400MHz, DMSO-d 6 )δ: 8.82(s, 1H), 8.29(s, 1H), 7.76(s, 1H), 7.32-7.43(m, 3H), 7.24(t, J=7.5Hz, 1H), 7.11-7.19(m ,2H),7.05(s,1H),6.61(d,J=7.5Hz,0H),4.71–4.64(m,1H),4.61(d,J=6.2Hz,2H),4.54–4.40(m, 1H), 3.69–3.57(m, 1H), 1.50(d, J=6.8Hz, 6H), 1.41(d, J=6.7Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com