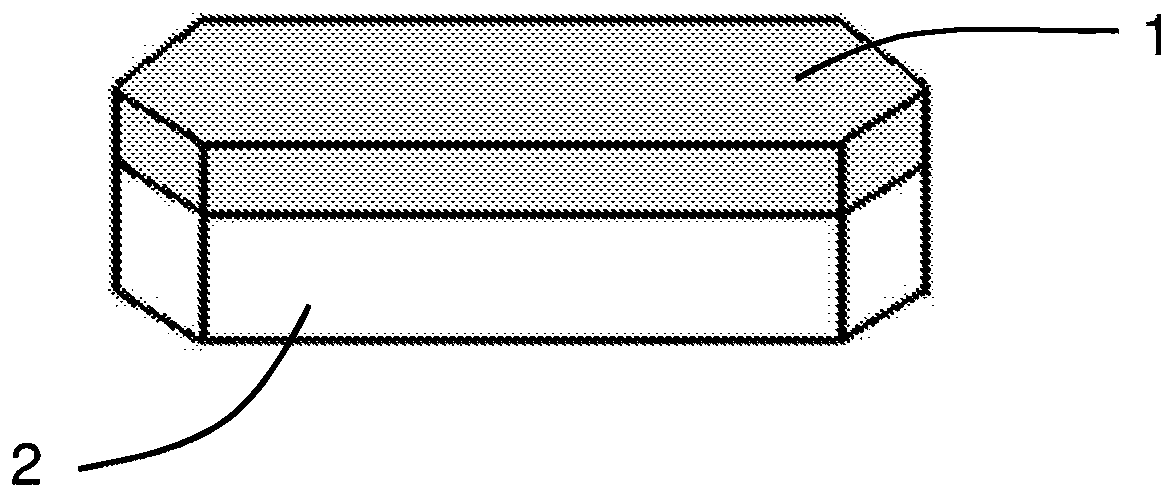
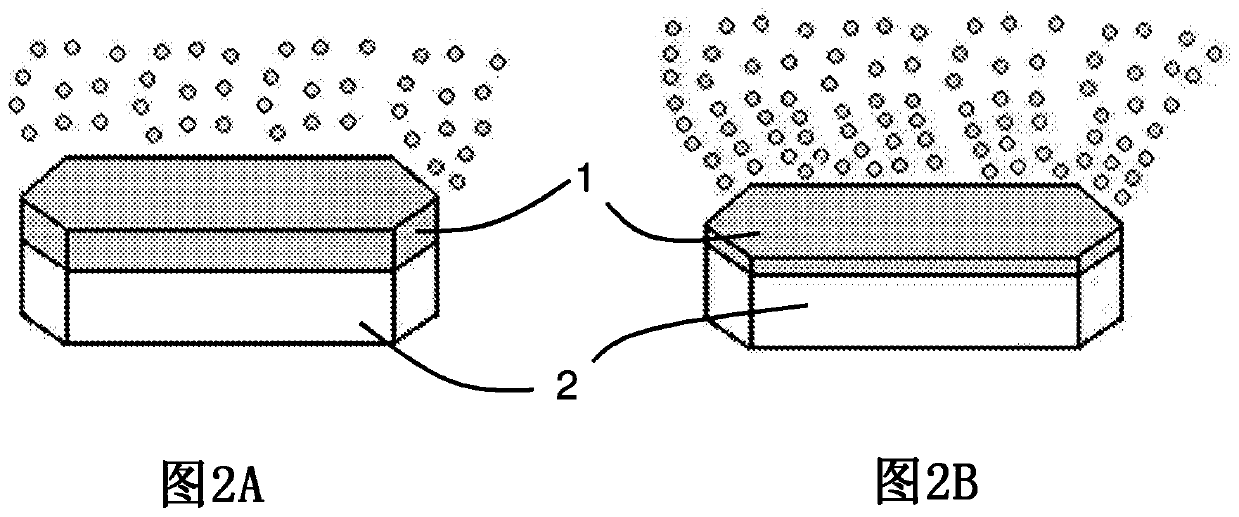
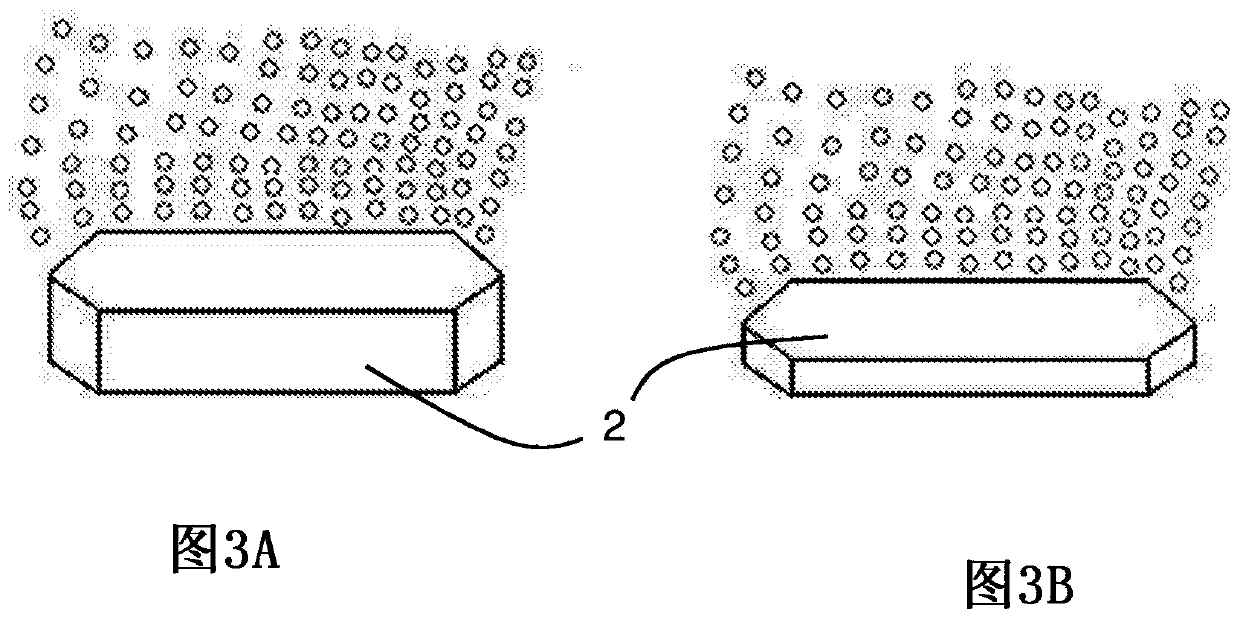
Pharmaceutical composition comprising 2,5-dihydroxybenzenesulfonic acid or a pharmaceutically acceptable salt thereof in the form of personalised supply units and corresponding manufacturing method
A technology of dihydroxybenzene sulfonic acid and supply unit, which is applied in the directions of drug combination, anhydride/acid/halide active ingredient, pill delivery, etc., can solve the problem of not being able to ensure immediate effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] The programmed release drug preparation process will be divided into the following two steps.
[0151] Step 1: Fabricate the Immediate Release Layer
[0152] a) 74.07% w / w calcium dobesilate, 20.74% w / w microcrystalline cellulose and 2.07% w / w sodium starch glycolate were sieved through a #20 sieve. All percentages are based on the total weight of the immediate release step.
[0153] b) Mixing the substances obtained in a) within a time interval of 1 minute to 30 minutes.
[0154] c) Sieve 1.33% w / w magnesium stearate and 1.78% w / w talc through a #30 sieve. All percentages are based on the total weight of the immediate release step.
[0155] d) Mixing the substances obtained in b) and c) within a time interval of 1 minute to 15 minutes.
[0156]
[0157] Step 2: Fabricate the Extended Release Layer
[0158] a) 53.76% w / w calcium dobesilate was micronized. All percentages are based on the total weight of the extended release step.
[0159] b) Dissolve 0.81%...
Embodiment 2
[0173] Step 1: Fabricate the Immediate Release Layer
[0174]
[0175] Step 2: Fabricate the Extended Release Layer
[0176]
[0177] * evaporates during the process
[0178] Both stages are compressed in the elongated punch. The ratio between the immediate release layer and the extended release layer was 31:69.
Embodiment 3
[0180] Step 1: Fabricate the Immediate Release Layer
[0181]
[0182] Step 2: Fabricate the Extended Release Layer
[0183]
[0184] * evaporates during the process
[0185] Both stages are compressed in the elongated punch. The ratio between the immediate release layer and the extended release layer is 34:66.
[0186] Figure 5 Release profiles of bilayer tablets are shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com