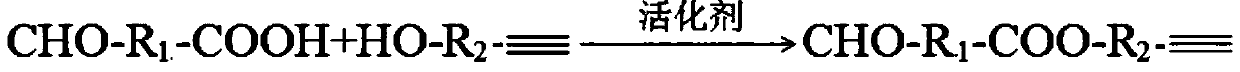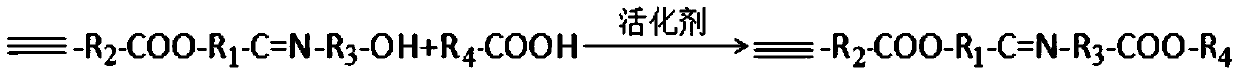pH-responsive nano preparation based on click reaction and preparation method and application thereof
A click-reaction, nano-formulation technology, which is applied in non-active ingredients medical preparations, medical preparations containing active ingredients, microcapsules, etc., can solve problems such as long biological half-life, reduce toxic and side effects, and achieve active targeting Sexuality, the effect of reducing the number of clinical injections and doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Preparation of gambogic acid-desulfated heparin derivative conjugate
[0069] Weigh p-formyl benzoic acid, propargyl alcohol and N,N-dimethyl-4-pyridinamine and place them in eggplant-shaped flasks. The molar ratio of propargyl alcohol to p-formyl benzoic acid is 1.2:1, N,N - The molar ratio of dimethyl-4-pyridinamine to p-formylbenzoic acid is 1:1, dissolve in 20mL of dichloromethane, seal the mixture with a rubber stopper, cool to 0°C, and stir at this temperature for 10min , DCC was added, the molar ratio of DCC to p-formylbenzoic acid was 1.5:1, the mixture was warmed to room temperature, and reacted overnight. The completeness of the reaction was monitored by TLC. After the reaction was complete, DCC was removed by suction filtration, the solvent methylene chloride was distilled off under reduced pressure, an appropriate amount of silica gel was added to make sand at 30°C, and intermediate I was obtained by separation and purification by column chromatog...
Embodiment 2
[0073] Example 2: Preparation of neogambogic acid-low molecular weight heparin derivative conjugate
[0074] Weigh glyoxylic acid, 3-butyn-1-ol, N,N-diisopropylethylamine and 4-pyrrolidinylpyridine into an eggplant-shaped bottle, 3-butyn-1-ol and acetaldehyde The molar ratio of acid is 2:1, the molar ratio of N,N-diisopropylethylamine to glyoxylic acid is 3:1, the molar ratio of 4-pyrrolidinylpyridine to glyoxylic acid is 2:1, and the In 20 mL of ethanol, the mixture was sealed with a rubber stopper, cooled to 0 ° C, and stirred at this temperature for 10 min, DCC was added, the molar ratio of DCC to glyoxylic acid was 1.5:1, the mixture was warmed to room temperature, and reacted overnight. The completeness of the reaction was monitored by TLC. After the reaction was complete, DCC was removed by suction filtration, the solvent ethanol was evaporated under reduced pressure, an appropriate amount of silica gel was added to make sand at 30°C, and intermediate I was obtained by sep...
Embodiment 3
[0078] Example 3: Preparation of ursolic acid-unfractionated heparin derivative conjugate
[0079] Weigh o-aldobenzoic acid, ethinylestradiol and 1-hydroxybenzotriazole and place them in eggplant-shaped bottles, the molar ratio of ethinylestradiol and o-aldobenzoic acid is 1.5:1, 1-hydroxybenzotriazole and The molar ratio of o-aldehydobenzoic acid is 1.5:1, dissolved in 20mL N,N-dimethylformamide, the mixture is sealed with a rubber stopper, cooled to 0°C, and stirred at this temperature for 10min, DCC is added, The molar ratio of DCC to o-aldehydobenzoic acid was 1.5:1, and the mixture was warmed to room temperature and reacted overnight. The completeness of the reaction was monitored by TLC. After the reaction was complete, DCC was removed by suction filtration, the solvent N,N-dimethylformamide was evaporated under reduced pressure, and an appropriate amount of silica gel was added to make sand at 30°C. Intermediate I was separated and purified by column chromatography. . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com