Preparation method of photo-repair azobenzene polymer
An azobenzene and light repair technology, applied in organic chemistry and other directions, can solve the problems of expensive Grubb catalyst, high price, complicated preparation process, etc., and achieve excellent photo-crosslinking and de-crosslinking characteristics, excellent characteristics under photoisomerization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of p-nitrophenoxyhexanol is prepared by reacting 6-chlorohexanol with p-nitrophenol.
[0043] 6-chlorohexanol (37.0mmol, 5.05g), 4-nitrophenol (44.4mmol, 6.18g), K 2 CO 3 (55.4mmol, 7.65g), KI (5mg), and DMF (50mL) were added to a three-necked flask, refluxed at 120 ° C, and the reaction was stopped after 6 hours of reaction. After drying, p-nitrophenoxyhexanol was obtained with a yield of about 87% and a purity of 95% by HPLC analysis.
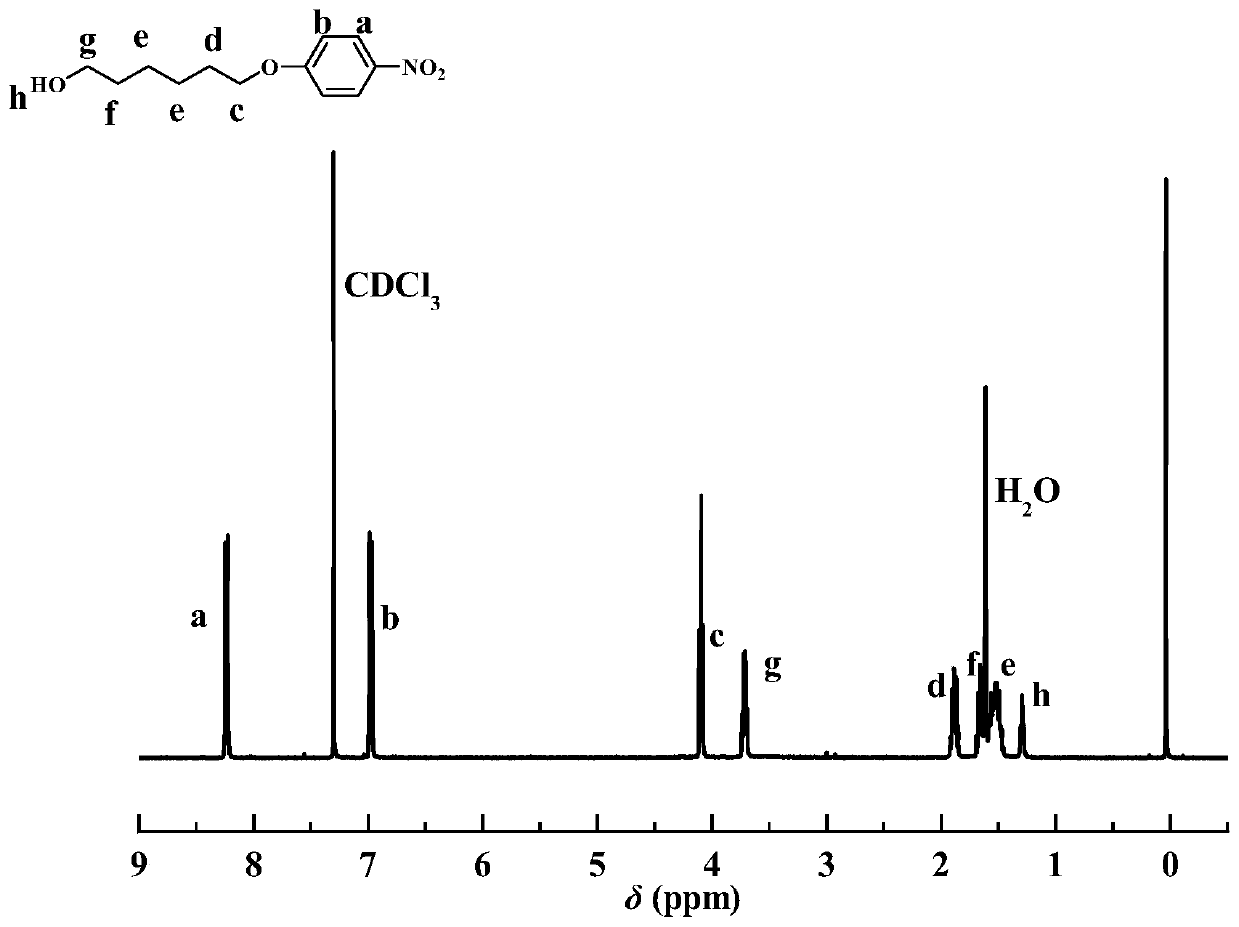
[0044] figure 1 It is the H NMR spectrum (CDCl) of purified p-nitrophenoxyhexanol 3 as a solvent). The peaks with chemical shifts of 8.08ppm and 6.99ppm correspond to the proton peaks (a, b) on the carbon of the benzene ring, and the peak with a chemical shift of 4.08ppm corresponds to the proton peak (c) on the methylene connected to oxygen, and the chemical shift is 3.72 The ppm peak corresponds to the proton peak (g) of the methylene group connected to the hydroxyl group, and the peaks with chemical shifts of 1.89...
Embodiment 2
[0046] Synthesis of p-nitrophenoxyhexanol
[0047] 6-chlorohexanol (22.2mmol, 3.03g), 4-nitrophenol (44.4mmol, 6.18g), K 2 CO 3 (55.4mmol, 7.65g), KI (5mg), and DMF (50mL) were added to a three-necked flask, refluxed at 120 ° C, and the reaction was stopped after 6 hours of reaction. After drying, p-nitrophenoxyhexanol was obtained with a yield of about 89% and a purity of 95% by HPLC analysis.
Embodiment 3
[0049] Synthesis of p-nitrophenoxyhexanol
[0050] 6-chlorohexanol (88.8mmol, 12.12g), 4-nitrophenol (44.4mmol, 6.18g), K 2 CO 3 (55.4mmol, 7.65g), KI (5mg), and DMF (50mL) were added to a three-necked flask, refluxed at 120 ° C, and the reaction was stopped after 6 hours of reaction. After drying, p-nitrophenoxyhexanol was obtained with a yield of about 85% and a purity of 96% by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com