A kind of high-dispersion ultra-small size carbon-supported noble metal catalyst and preparation method thereof
A noble metal catalyst, small size technology, applied in the preparation of high dispersion ultra-small size carbon-supported noble metal catalyst, the field of high-dispersion ultra-small size carbon-supported noble metal catalyst, can solve the limitation of the widespread application of fuel cells, the large diameter of platinum particles , limited reserves, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
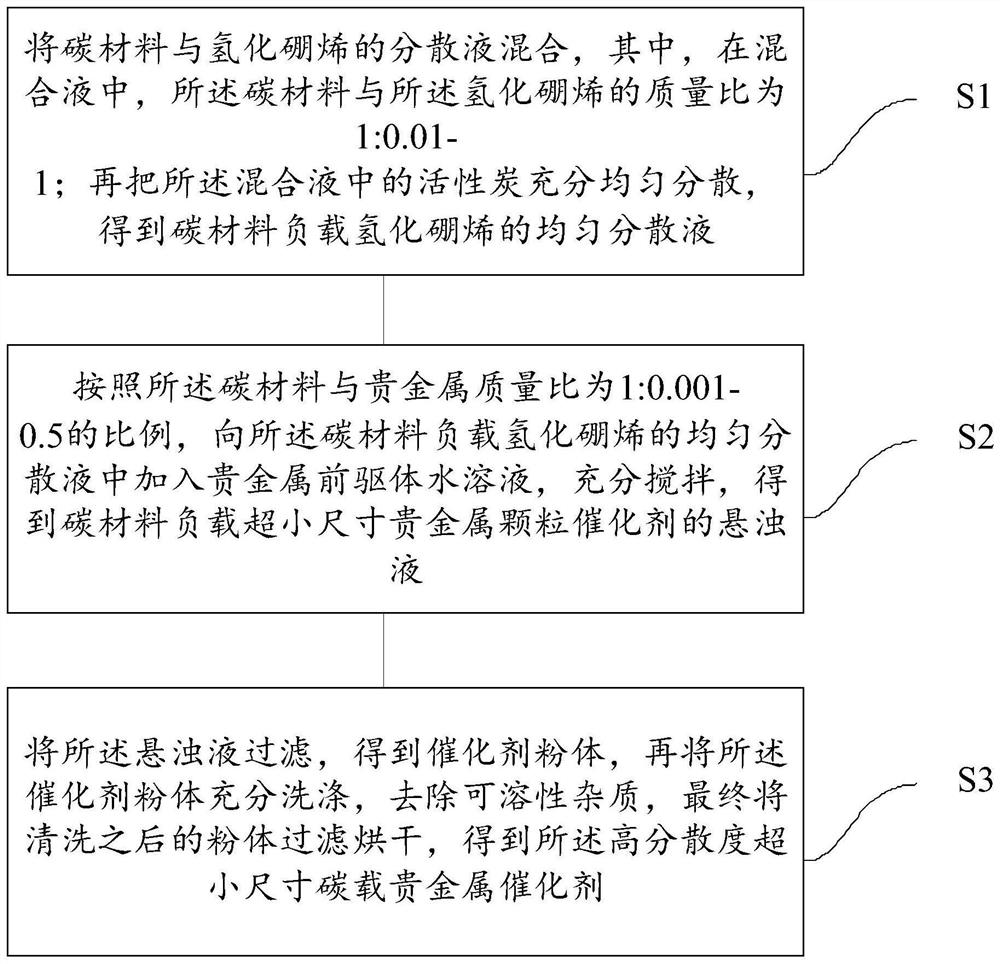
[0033] refer to figure 1 , shows a step flow chart of a method for preparing a high-dispersion ultra-small size carbon-supported noble metal catalyst of the present invention, and the method may specifically include the following steps:
[0034] Step S1: Mix the carbon material with the dispersion of borolene hydride, wherein, in the mixed solution, the mass ratio of the carbon material to the borohydride is 1:0.01-1; Activated carbon is fully and evenly dispersed to obtain a uniform dispersion of carbon material-supported boron hydride;
[0035] In the present invention, the above-mentioned carbon material may be one or more of carbon black, activated carbon, graphene, graphene oxide, graphyne, carbon nanotubes, carbon nanofibers, carbon nanospheres, natural graphite and porous carbon.
[0036] The solvent used in the dispersion of borohydride can be one or more of methanol, ethanol, acetone, tetrahydrofuran, and N,N-dimethylformamide. Among them, the mass concentration of ...
Embodiment 1
[0047] Experiments were performed on the precious metal "platinum".
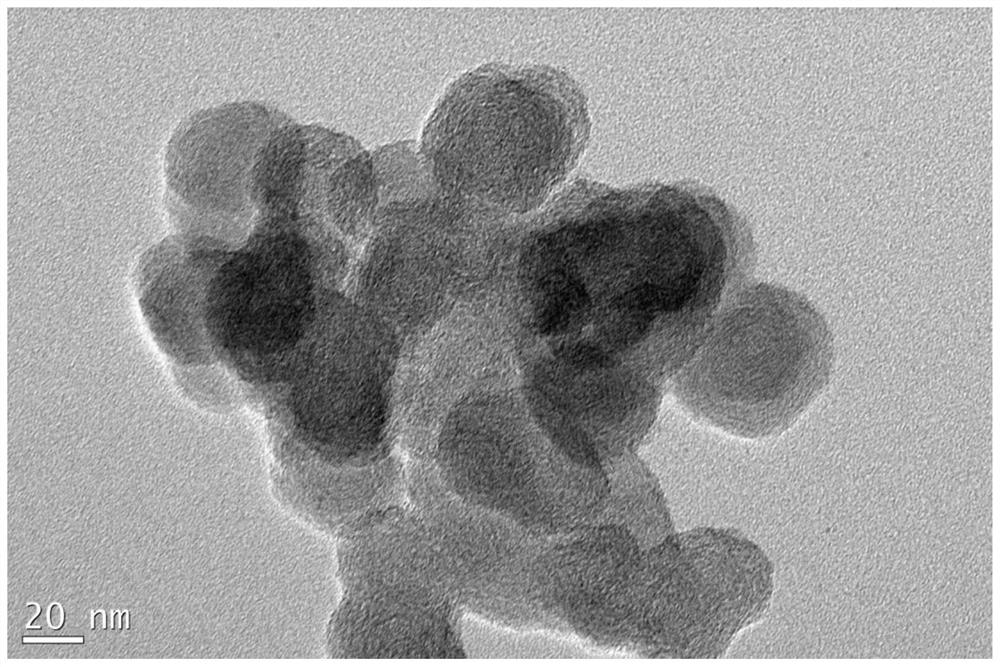
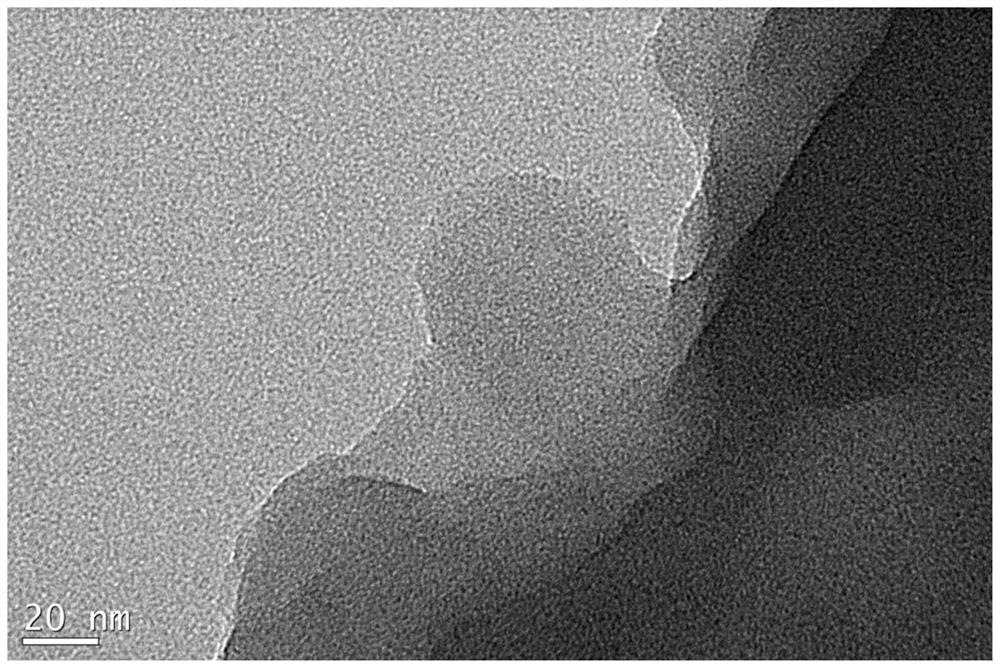
[0048] First, weigh 25 mg of activated carbon, and mix the activated carbon with 1.25 mL of borohydride methanol dispersion, wherein the mass concentration of borohydride methanol dispersion is 20 mg / mL, and the mass ratio of activated carbon to borohydride is 1:1; Then, magnetic stirring and ultrasonic dispersion are used to fully and uniformly disperse the activated carbon to obtain a uniform dispersion of activated carbon-supported boron hydride. Next, according to the mass ratio of activated carbon and precious metal mass ratio of 1:0.5, add 315 μl potassium chloroplatinate aqueous solution to the above dispersion liquid, wherein the concentration of potassium chloroplatinate aqueous solution is 200mM (1mM=1mmol / L, in the following examples , all expressed in mM as the concentration unit of the noble metal aqueous solution), fully stirred at room temperature to obtain a suspension of activated carbon sup...
Embodiment 2
[0051] Experiments were performed on the precious metal "gold".
[0052] First, take 25mg of activated carbon, mix the activated carbon with 20ml of borolene ethanol dispersion, the mass concentration of borolene ethanol dispersion is 0.25mg / ml, wherein the mass ratio of activated carbon to borolene is 1:0.2; then , using magnetic stirring and ultrasonic dispersion to fully and uniformly disperse the activated carbon to obtain a uniform dispersion of activated carbon-supported boron hydride. Then, according to the mass ratio of activated carbon to precious metal mass ratio of 1:0.125, add 320 μl of chloroauric acid aqueous solution to the above dispersion liquid, wherein the concentration of chloroauric acid aqueous solution is 100mM, and fully stir at room temperature to obtain activated carbon-loaded ultra-small size gold nanoparticles Suspension of particulate catalyst. The above-mentioned suspension is filtered to obtain a solid precipitate, which is fully washed with wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com