Synthesis method of 2-formyl-4-carboxylic acid ethyl thiazole
A technology for the synthesis of ethyl thiazole carboxylate, which is applied in the chemical and pharmaceutical fields, can solve the problems of poor economy, complicated operation, and high production cost, and achieve the effects of low cost, high chemical purity, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] Example 1
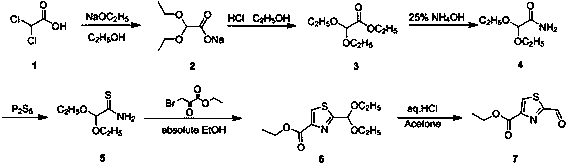
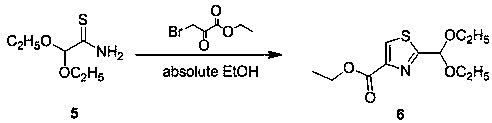
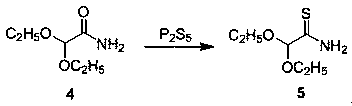
[0033] A synthetic method of 2-formyl-4-carboxylic acid ethyl thiazole, such as figure 1 As shown, including the following steps:
[0034] The first step is to react 2,2-dichloroacetic acid (1) with sodium ethoxide to produce sodium 2,2-diethoxy acetate (2):
[0035] Add the raw material 2,2-dichloroacetic acid (1) to the ethanol solution of sodium ethoxide, the molar ratio of 2,2-dichloroacetic acid (1) to sodium ethoxide is 1:3, and at 60-80 ℃, Stir for 2-3 h until the reaction is completed to obtain sodium 2,2-diethoxyacetate (2); the reaction solution is directly used in the next reaction without treatment.
[0036] The second step is to react sodium 2,2-diethoxy acetate (2) with an ethanol solution of hydrogen chloride to obtain ethyl 2,2-diethoxy acetate (3):
[0037] At 0-5 ℃, the ethanol solution of hydrogen chloride was added dropwise to the reaction solution of the first step, the molar ratio of hydrogen chloride to 2,2-diethoxy sodium acetate (2) was 1:1....
Example Embodiment
[0047] Example 2
[0048] A synthetic method of 2-formyl-4-carboxylic acid ethyl thiazole, such as figure 1 As shown, including the following steps:
[0049] The first step: reacting 2,2-dichloroacetic acid (1) with sodium ethoxide to produce sodium 2,2-diethoxyacetate (2);
[0050] The raw material 2,2-dichloroacetic acid (1) is added to the ethanol solution of sodium ethoxide, the molar ratio of 2,2-dichloroacetic acid (1) to sodium ethoxide is 1:3.5, and under the condition of 60-80 ℃, Stir for 2-3 h until the reaction is completed to obtain sodium 2,2-diethoxyacetate (2); the reaction solution is directly used in the next reaction without treatment.
[0051] The second step: reacting sodium 2,2-diethoxy acetate (2) with an ethanol solution of hydrogen chloride to obtain ethyl 2,2-diethoxy acetate (3);
[0052] Under the condition of 0-5℃, the ethanol solution of hydrogen chloride was added dropwise to the reaction solution of the first step. The molar ratio of hydrogen chloride to...
Example Embodiment
[0061] Example 3
[0062] A synthetic method of 2-formyl-4-carboxylic acid ethyl thiazole, such as figure 1 As shown, including the following steps:
[0063] The first step: reacting 2,2-dichloroacetic acid (1) with sodium ethoxide to produce sodium 2,2-diethoxyacetate (2);
[0064] Add the raw material 2,2-dichloroacetic acid (1) to the ethanol solution of sodium ethoxide, the molar ratio of 2,2-dichloroacetic acid (1) to sodium ethoxide is 1:4, and at 60-80 ℃, Stir for 2-3 h until the reaction is completed to obtain sodium 2,2-diethoxyacetate (2); the reaction solution is directly used in the next reaction without treatment.
[0065] The second step: reacting sodium 2,2-diethoxy acetate (2) with an ethanol solution of hydrogen chloride to obtain ethyl 2,2-diethoxy acetate (3);
[0066] At 0-5 ℃, the ethanol solution of hydrogen chloride was added dropwise to the reaction solution of the first step, the molar ratio of hydrogen chloride to 2,2-diethoxy sodium acetate (2) was 1:2.05; a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com