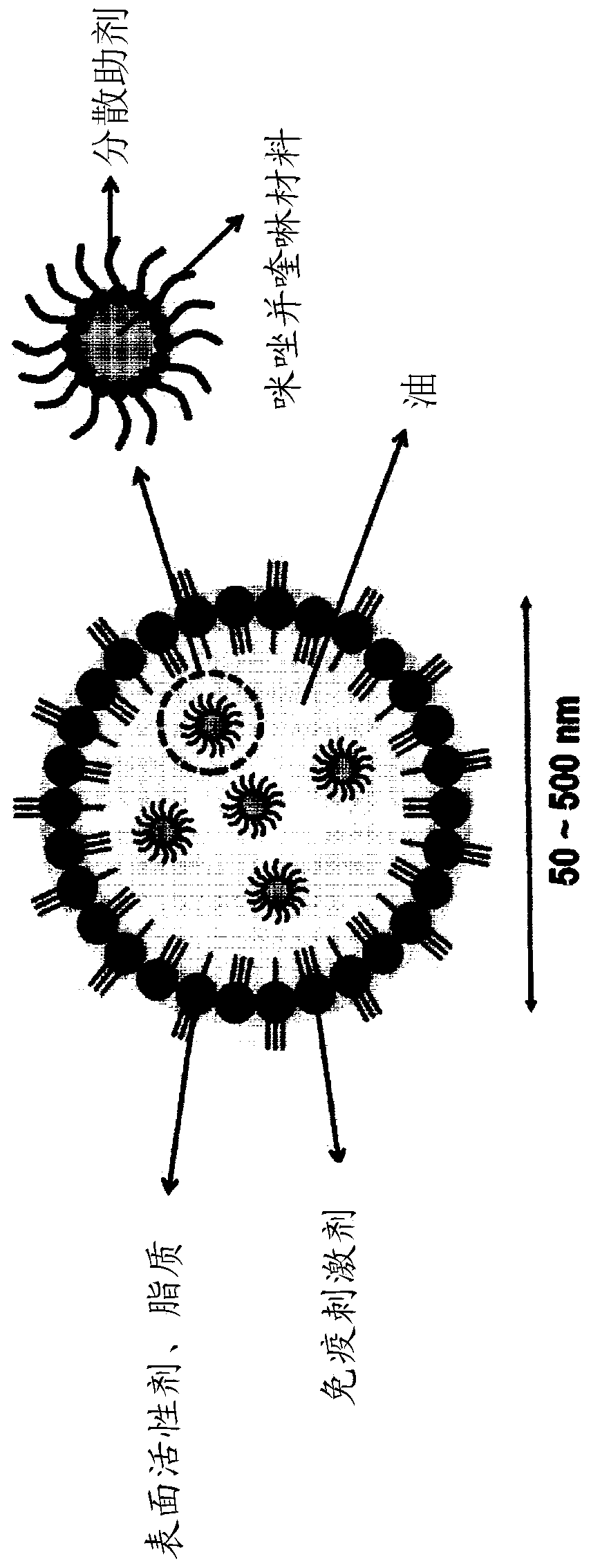
Nanoemulsion comprising imidazoquinoline-based material and use thereof
A technology of imidazoquinoline and nanoemulsion, which is applied in the field of adjuvants or vaccines, can solve problems such as inappropriateness, toxicity and side effects, and achieve the effect of enhancing cellular immunity and solving systemic toxicity problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1. Preparation of squalene solution comprising imiquimod (R837)
[0093] 20 mg of imiquimod (TCI, Tokyo, Japan) was treated at room temperature with a bath sonicator (Branson MH Mechanical Bath 5800, Emerson, St. Louis MO) was dissolved in 100 mg oleic acid (SigmaAldrich, USA) until the solution became transparent. Thereafter, 1 mL of squalene (5% v / v, Sigma Aldrich, USA) was added thereto and stirred using a stirrer (Vortex-Genie 2, Scientific Industries, USA) to obtain a uniform squalene solution.
Embodiment 2
[0094] Example 2. Preparation and Characterization of Squalene Nanoemulsions (NE-IQ) Including Imiquimod
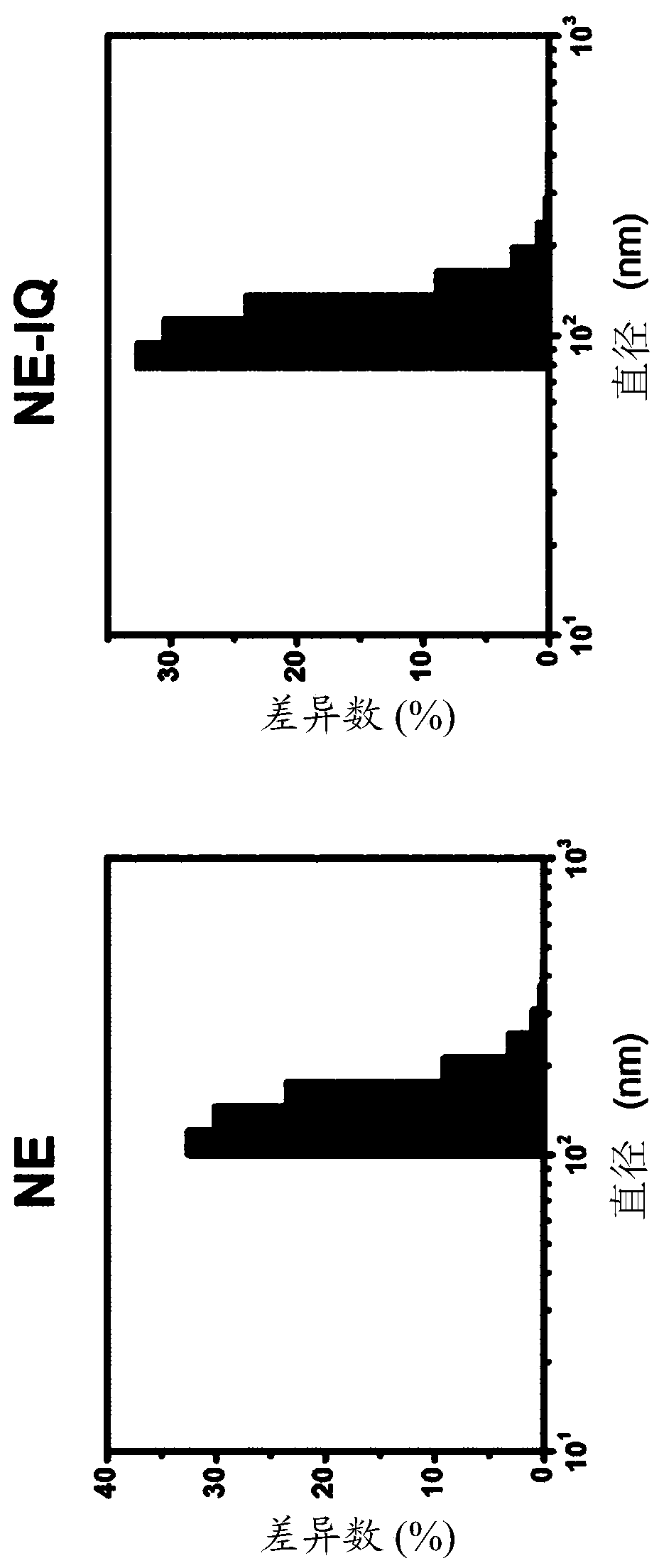
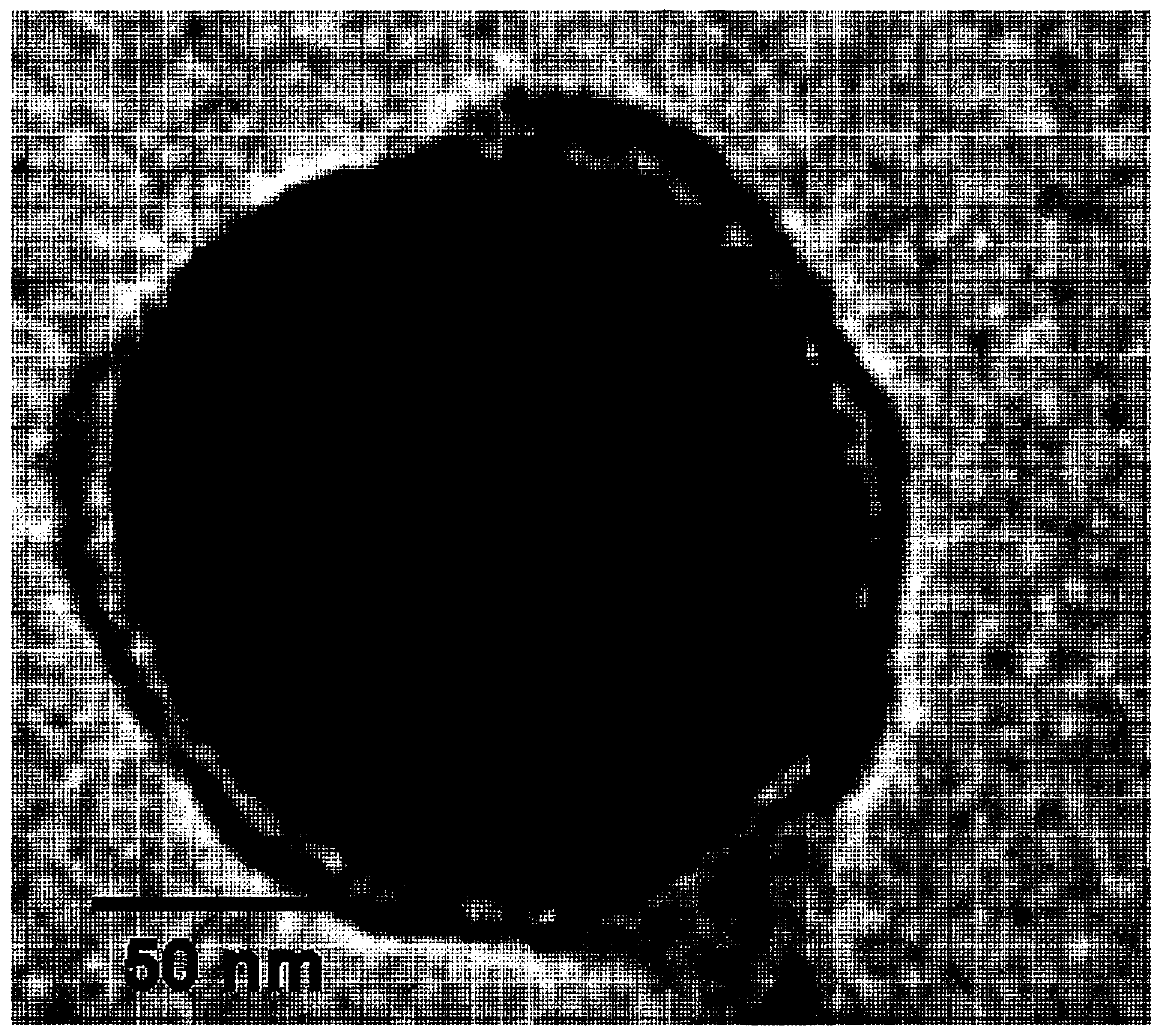
[0095] Squalene (5% v / v, Sigma Aldrich, USA) containing imiquimod prepared according to Example 1, Tween 80 (0.5% v / v, Sigma Aldrich, USA) and Span 85 (0.5% v / v, Sigma Aldrich, USA) was dissolved in 2mL phosphate buffered saline (PBS, 0.0067M PO 4 ), and then completely dispersed in phosphate buffered saline (PBS) for 1 min using an ultrasonic disperser (Tip sonicator). Then, the mixture was stirred using a tube rotator for about 2 h, and then stored in a refrigerator at 4 °C until use. The size of the emulsion was analyzed by dynamic light scattering (DLS, Otsuka, Japan). As a result of dynamic light scattering (DLS) measurement, diameters of 106.16±5.4 and 148.54±18.5 nm were confirmed (see FIG. 2 and Table 1).
[0096] [Table 1]
[0097]
Embodiment 3
[0098] Example 3. Introduction of NE-IQ into immune cells and its localization in cells
[0099] Bone marrow-derived dendritic cells (BMDCs) and bone marrow-derived macrophages (BMMCs) were treated with squalene containing imiquimod (R837) for 24 hours and then evaluated using fluorescence microscopy. Such as image 3 As shown in the fluorescence image of , lysotracker (green) and DID (red) were found to co-localize at the same location in the cell. From these results, it was confirmed that the squalene nanoemulsion containing imiquimod (NE-IQ) was localized in endosomes and lysosomes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com