Planar microwave cavity drip-in type liquid dielectric substance value detection sensor
A microwave cavity and dielectric technology, which is applied in the direction of material analysis, instruments, and measurement devices using microwave means to achieve the effect of simple processing circuits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
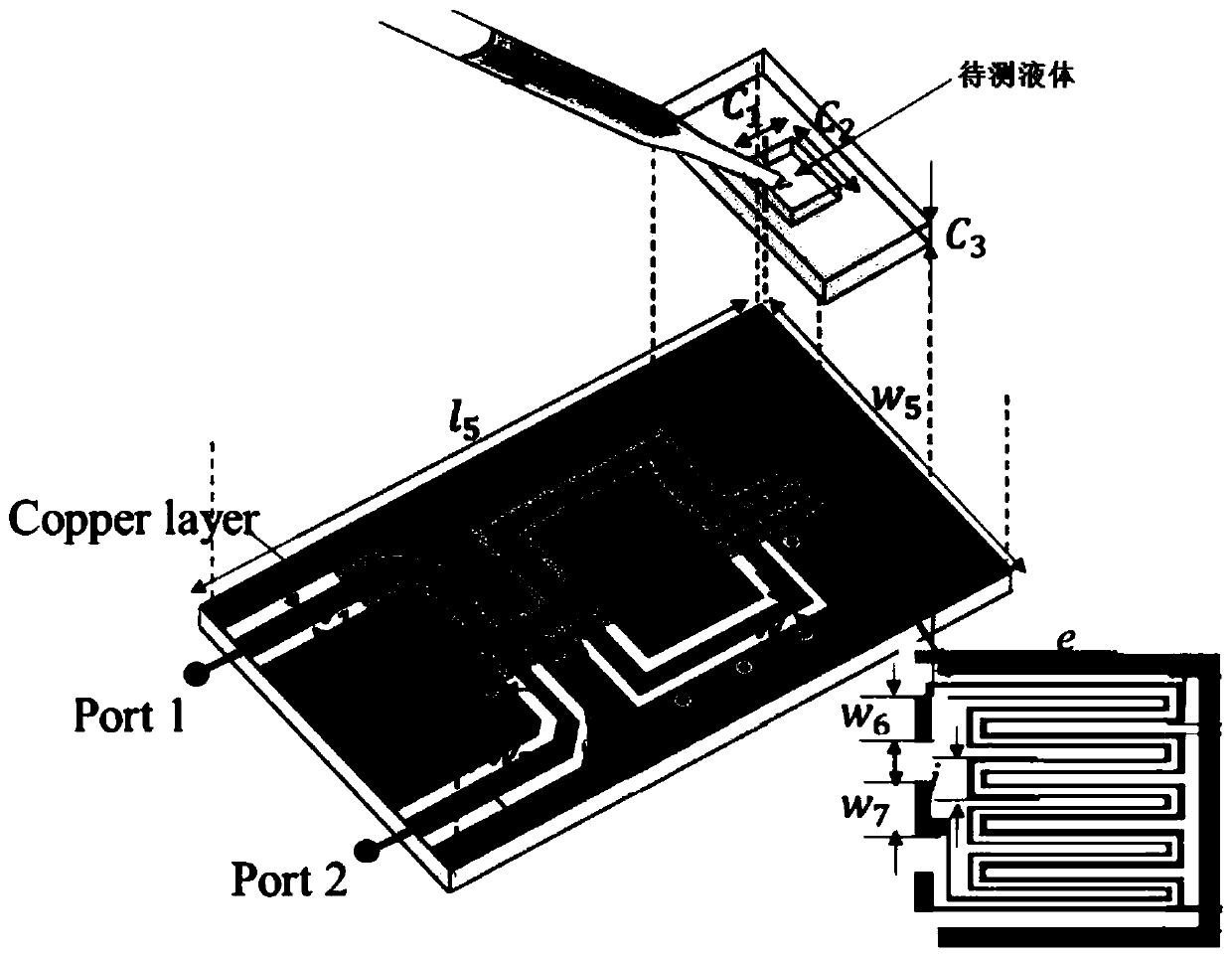
[0028] The inner dimension of the FR4 container that the present invention adopts is 7.5 * 7.5mm 2 , to cover the sensor detection area. The height of the container is 1.6 mm, which provides enough space for adjusting the volume of the liquid to be measured. The width and length of the container (ie c1 and c2) are respectively larger than the width of the detection area sensor (4.1×4.6mm 2 ) 3.4 and 2.9mm larger to ensure that the liquid to be tested is exposed to the electric field on the side of the container.
[0029] A parametric study of the influence of the volume of the liquid to be measured on the reflected notch frequency and decibels is carried out. different volumes of liquid to be tested for the evaluation of the sensor, Figure 5 Displays the measurement results. S11 was evaluated for different volumes of 0.02, 0.04, 0.06, 0.08 and 0.1 mL of deionized (DI) water placed on the detection area. Deionized water was chosen because it has a high dielectric constant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com