A liquid chromatography-mass spectrometry screening method for multi-target antithrombotic active substances
A screening method and anti-thrombotic technology, applied in the field of liquid chromatography-mass spectrometry screening, can solve the problems of affecting the sensitivity of mass spectrometry, increasing operating steps, increasing reagent costs, etc., to save analysis time, strong anti-interference ability, and save reagent costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
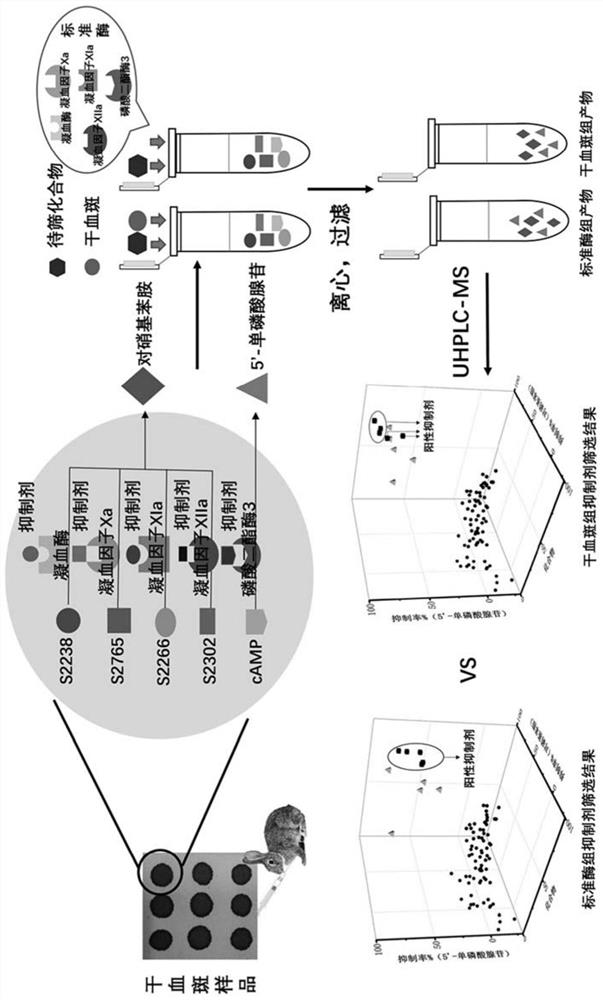
[0044] The present invention obtains a liquid chromatography-mass spectrometry screening method for multi-target antithrombotic active substances based on dried blood spot samples through the following steps:
[0045] 1. Dried blood spot sample preparation and standard solution preparation :
[0046] 1.1 Preparation of dried blood spot samples: pipette 10-80 μL of whole blood onto a Whatman903 filter paper card, and air-dry at room temperature for 2 hours to obtain a dried blood spot sample. The prepared dried blood spot sample is placed in a sealed bag containing a desiccant and stored at -20°C for later use.
[0047] 1.2 Preparation of substrate standard solution: Accurately weigh an appropriate amount of substrate powder, and use deionized water to prepare substrates S2238, S2765, S2266, S2302 and cyclic adenosine monophosphate (cAMP) to 500mg L -1 Concentrated solutions are stored.
[0048] 1.3 Preparation of the compound solution to be screened: 78 compounds to be scr...
Embodiment 2
[0070] The method validation of the established product quantification method, and the investigation of the quantification limit, detection limit, precision, accuracy and matrix effect, should meet the requirements of the 2015 edition of the Chinese Pharmacopoeia IV "Guiding Principles for Validation of Analytical Methods".
[0071] (1) Quantitation limit and detection limit
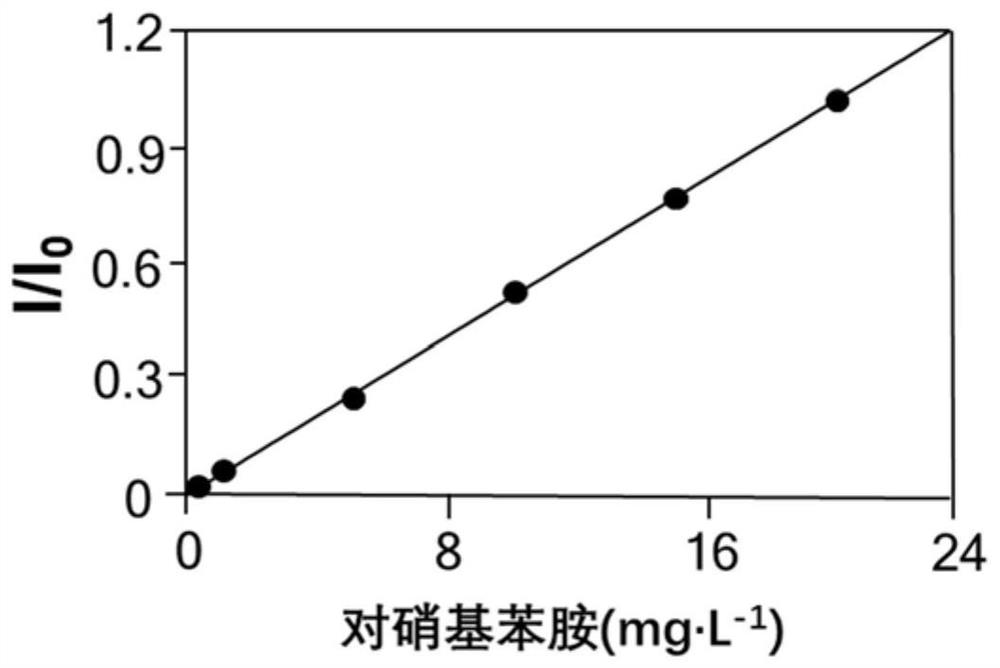
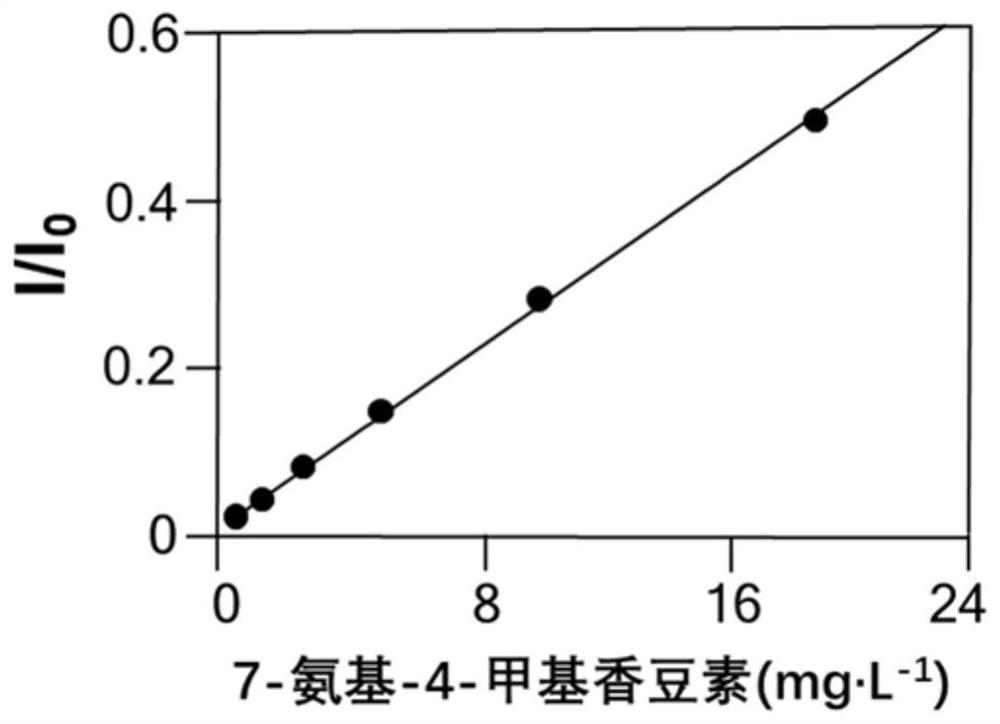
[0072] Detection limit of p-nitroaniline: 5 μg L -1 (S / N=3.3±1.61; n=6); lower limit of quantitation: 10 μg L -1 (S / N=10.5±1.86; n=6); detection limit of 7-amino-4-methylcoumarin: 4 μg L -1 (S / N=1.4±2.71; n=6); lower limit of quantitation: 10 μg L -1 (S / N=12.3±1.71; n=6); detection limit of 5’-adenosine monophosphate: 5 μg L -1 (S / N=3.76±1.37; n=6); lower limit of quantitation: 10 μg L -1 (S / N=10.89±1.69; n=6); detection limit of 5’-guanosine monophosphate: 8 μg L -1 (S / N=6.62±2.39; n=6); lower limit of quantitation: 15 μg L -1 (S / N=13.66±1.52; n=6). From the results, it can be seen that the detec...
Embodiment 3
[0086] Using standard enzymes to verify the liquid chromatography-mass spectrometry screening method for multi-target antithrombotic active substances:
[0087] 1. Solution preparation: Accurately weigh appropriate amount of thrombin, coagulation factor Xa, coagulation factor XIa, coagulation factor XIIa, and phosphodiesterase 3 powders, and dilute them to 1000U L with deionized water -1 concentration and stored at -20°C for use; accurately weigh appropriate amounts of substrates S2238, S2765, S2266, S2302, and cAMP powder, and prepare 500mg L with deionized water -1 Concentration solutions were stored; 78 compounds to be screened were respectively taken, dissolved in a small amount of DMSO, and prepared as 100 μM stock solutions in water. The above stock solutions were stored at -20°C in the dark. Dissolve argatroban, rivaroxaban, benzamidine, 3-acetylcoumarin, and milrinone in 50 µL of DMSO, respectively, and then add water to a final concentration of 500 µM for storage. W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com