A kind of injectable natural triterpenoid antibacterial hydrogel and preparation method thereof
A triterpene compound, injection-type technology, applied in the directions of antibacterial drugs, topical antibacterial agents, drug combinations, etc., can solve the problems of increased preparation cost and low application value, and achieves good bacteriostatic effect and high mechanical strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
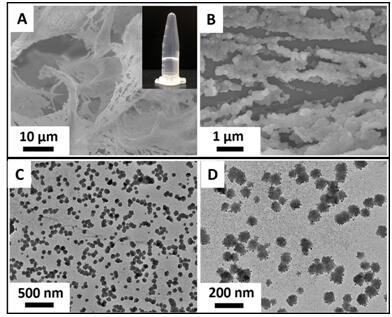
[0031] After in-depth research, the inventors prepared a triterpenoid hydrogel with inherent antibacterial properties and injectable properties. The present invention makes full use of the antibacterial properties and amphiphilic molecular structure of natural triterpenoids, and selects hydrogels with injectable and antibacterial properties through gelation experiments and research on antibacterial, cell activity and hemolysis experiments of hydrogels and preparation method.
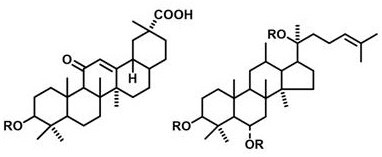
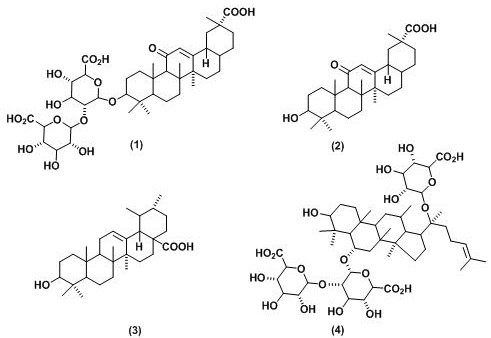
[0032] In this example, a mixture of glycyrrhizic acid and glycyrrhetinic acid in pentacyclic triterpenoids is used as a gel molecule to prepare a hydrogel, and a PBS buffer with pH=7 is used as a solvent. Wherein, the mole fraction of glycyrrhizic acid is 70%, and the mole fraction of glycyrrhetic acid is 30%, and the specific preparation method is as follows:
[0033] First, a suspension solution of glycyrrhizic acid and glycyrrhizic acid with a concentration of 3.5 mM was prepared in a container, in ...
Embodiment 2
[0035] In this example, a mixture of glycyrrhizic acid and ursolic acid in pentacyclic triterpenoids is used as a gel molecule to prepare a hydrogel, and a PBS buffer with pH=7.4 is used as a solvent. Wherein, the mole fraction of glycyrrhizic acid is 60%, and the mole fraction of ursolic acid is 40%, and the specific preparation method is as follows:
[0036] First, prepare a suspension solution of glycyrrhizic acid and ursolic acid with a concentration of 5mM in a container, in which the mole fraction of glycyrrhizic acid is 60% and the mole fraction of ursolic acid is 40%; The solution was ultrasonically treated for 2 min to promote the uniform dispersion of glycyrrhizic acid and ursolic acid molecules in the aqueous solution; then the suspension solution was placed in an oven at 97 °C and heated for 150 min, at which time the solution changed from a turbid state to a transparent state; finally, the transparent solution was It was placed in an environment of 25° C. until th...
Embodiment 3
[0038] In this example, a mixture of glycyrrhizic acid and ginsenosides in tetracyclic triterpenoids is used as a gel molecule to prepare a hydrogel, and a PBS buffer with pH=7.2 is used as a solvent. Wherein, the mole fraction of glycyrrhizic acid is 90%, and the mole fraction of ginsenoside is 10%, and the specific preparation method is as follows:
[0039]First, a mixed suspension solution of 2mM glycyrrhizic acid and ginsenoside was prepared in a container with a pH=7.2 PBS buffer, wherein the mole fraction of glycyrrhizic acid was 90% and the mole fraction of ginsenoside was 10%; then at 37°C The suspension was ultrasonically treated for 15 min under the same environment to promote the two gel molecules to disperse evenly in the aqueous solution; then the suspension solution was placed in an oven at 90 °C and heated for 120 min, at which time the solution changed from a turbid state to a transparent state; finally The transparent solution was placed in an environment of 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com