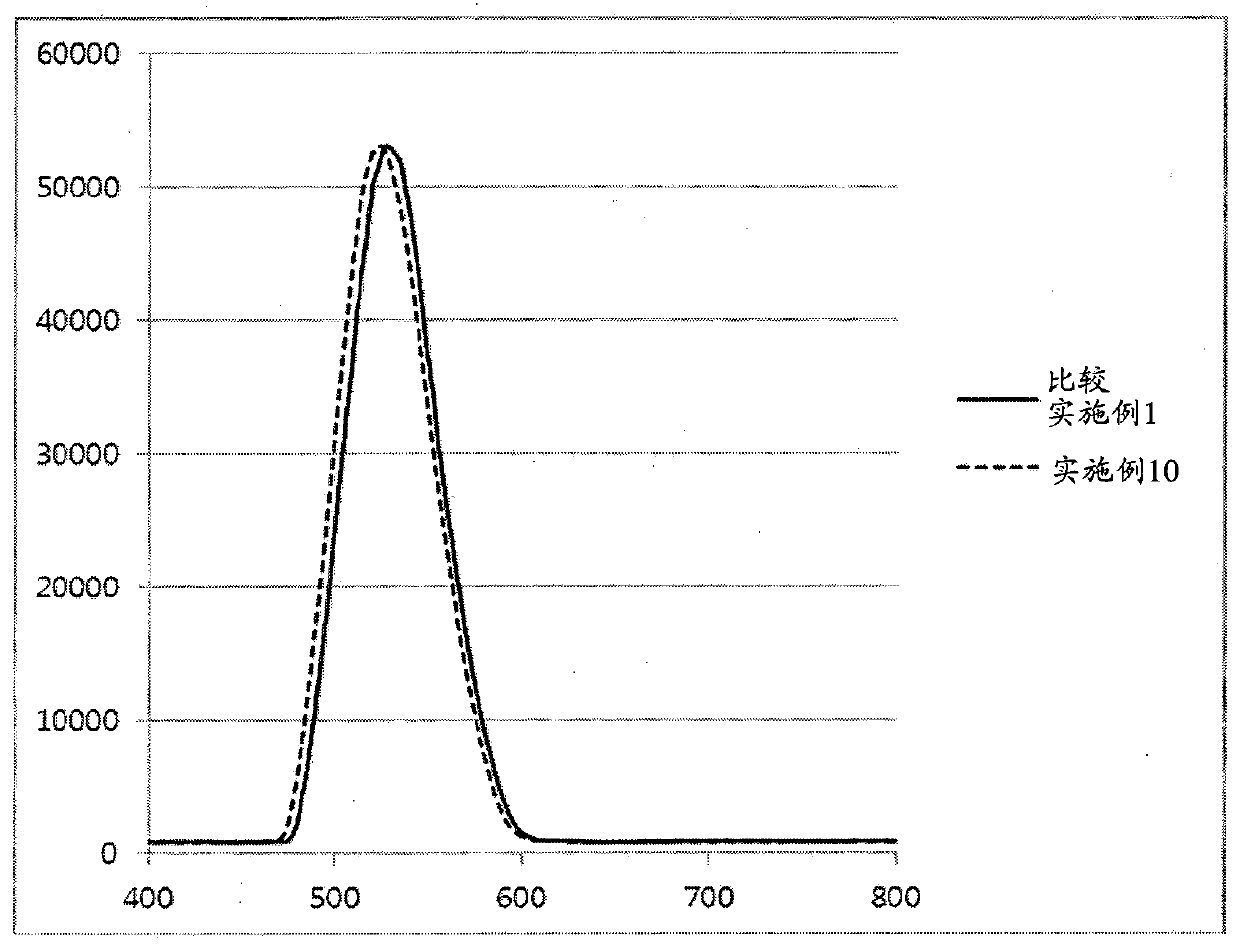
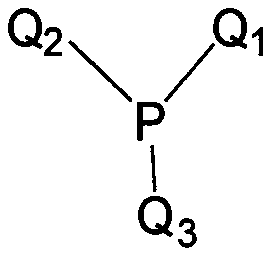
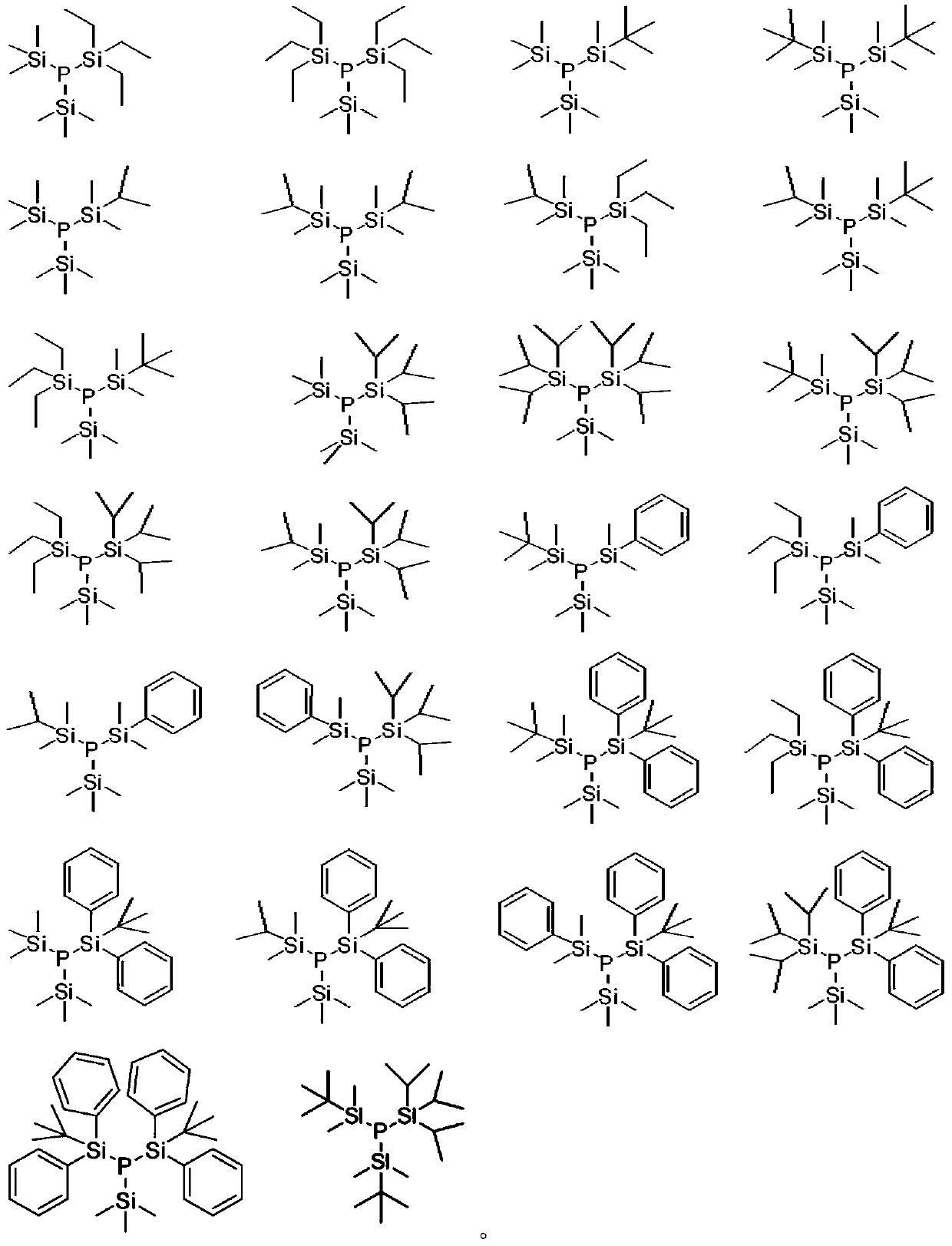
Phosphine precursor for preparing quantum dot and quantum dot prepared therefrom
一种量子点、前体的技术,应用在用于制备量子点的膦前体以及由膦前体制备的量子点领域,能够解决降低半峰全宽等问题,达到高发光颜色纯度、改善发光效率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0105] A preparation method in which a secondary phosphine compound is used as a starting material is shown in Equation 3 below:
[0106] [reaction formula 3]
[0107]
[0108] In Equation 3,
[0109] Q 1 to Q 3 as defined in Formula 1, and X 1 means halogen.
[0110] The preparation method in which the primary phosphine compound is used as the starting material is shown in the following reaction scheme 4:
[0111] [Reaction 4]
[0112]
[0113] In Equation 4,
[0114] Q 1 to Q 3 as defined in Formula 1, and X 1 and x 2 Each of independently means halogen.
[0115] Hereinafter, materials used in the preparation method will be explained in detail.
[0116] As the ether solvent, tetrahydrofuran (THF) which does not exhibit reactivity with other raw materials can be used.
[0117] As the alkyllithium, butyllithium (BuLi) can be used.
[0118] Halogenated hydrocarbons are generally polar and well known as non-flammable materials, but are not used as reaction solv...
Embodiment 1
[0154] Example 1: (tert-butyldimethylsilyl)bis(trimethylsilyl)phosphine (t-BuMe 2 Si)(Me 3 Si) 2 P Synthesis
[0155]
[0156] Step 1: Preparation of bis(trimethylsilyl)phosphine
[0157] In a 2L round-bottom three-neck flask under a nitrogen atmosphere, 30 g of tris(trimethylsilyl)phosphine was added to 500 ml of dichloromethane, and the mixture was cooled to 0°C. While keeping the temperature at 0°C, 3.8 g of methanol were added dropwise for 1 hour. The bis(trimethylsilyl)phosphine produced was used in subsequent processes without a separate purification process.
[0158] Step 2: Preparation of (tert-butyldimethylsilyl)(trimethylsilyl)phosphine
[0159] Dilute the bis(trimethylsilyl)phosphine prepared in step 1 in 200ml of MC, and add 35.4g of Et 3 N was cooled to 0° C., and then 32.7 g of tert-butyldimethylsilyl triflate were added dropwise for 1 hour. The obtained reactant was vacuum distilled without filtration to obtain 15.1 g of the target compoun...
Embodiment 2
[0163] Example 2: Bis(tert-butyldimethylsilyl)(trimethylsilyl)phosphine (t-BuMe 2 Si) 2 (Me 3 Si)P Synthesis
[0164]
[0165] Step 1: Preparation of Trimethylsilylphosphine
[0166] In a 2L round-bottom three-neck flask under a nitrogen atmosphere, 30 g of tris(trimethylsilyl)phosphine was added to 500 ml of dichloromethane, and the mixture was cooled to 0°C. While keeping the temperature at 0°C, 7.6 g of methanol were added dropwise for 1 hour. The trimethylsilylphosphine produced is used in subsequent processes without a separate purification process.
[0167] Step 2: Preparation of bis(tert-butyldimethylsilyl)(trimethylsilyl)phosphine
[0168] Dilute the trimethylsilylphosphine prepared in step 1 in 200ml of MC, and add 70.8g of Et 3 N was cooled to 0° C., and then 62.4 g of tert-butyldimethylsilyl triflate were added dropwise for 1 hour. The obtained reactant was vacuum distilled without filtration to obtain 10.2 g of the target compound (tert-butyld...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com