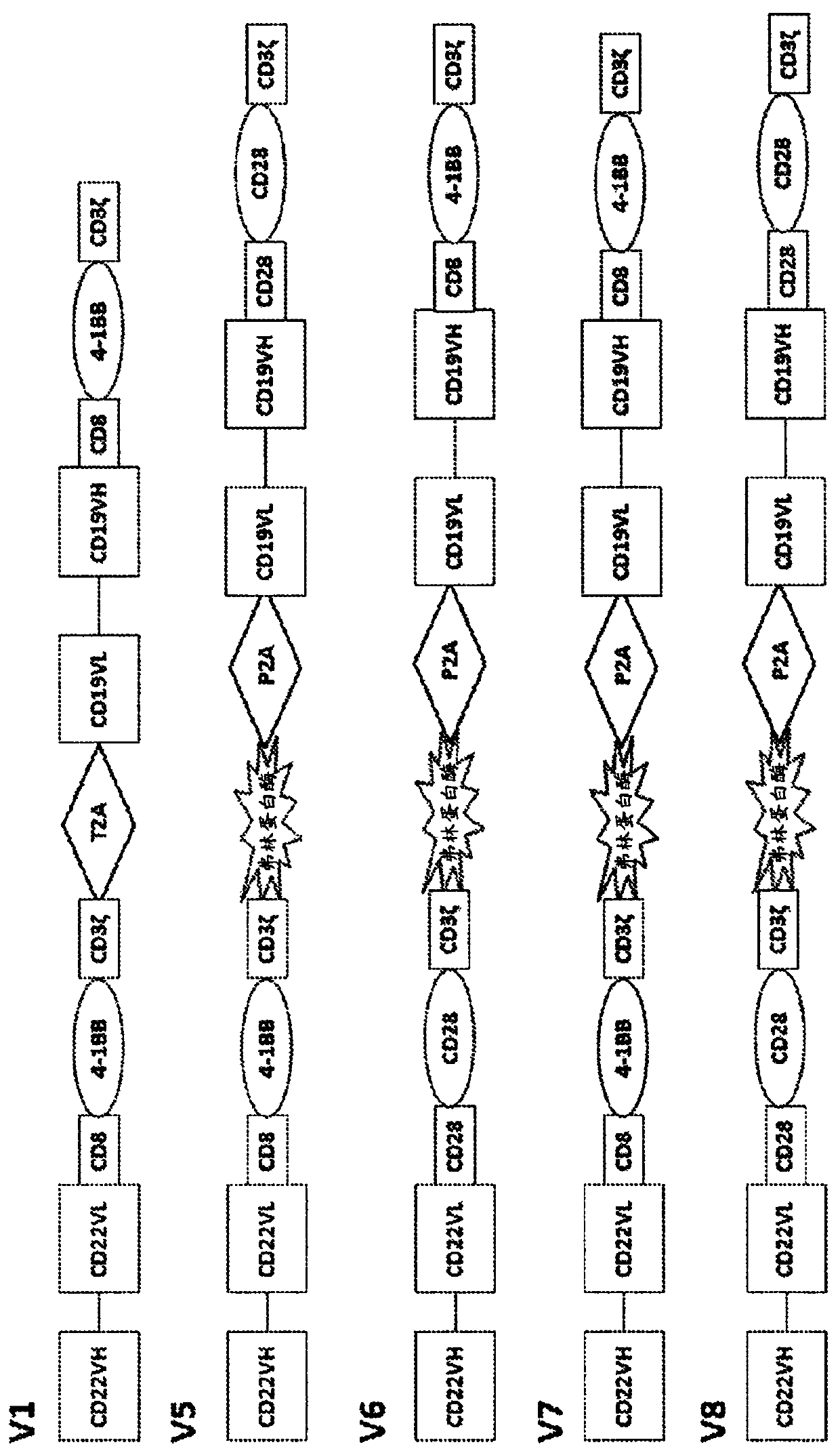
Bicistronic chimeric antigen receptors and their uses
A chimeric antigen receptor, antigen technology, applied in the direction of antibodies, antibody mimics/scaffolds, receptors/cell surface antigens/cell surface determinants, etc., can solve the problems of unmet cancer treatment and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0300] This example demonstrates the production of CAR constructs, lentiviral vectors encoding the CAR constructs, and CAR-expressing T cells according to embodiments of the invention, as well as the production of other CARs for comparison.
[0301] The CAR construct was synthesized by GENEWIZ (South Plainfield, NJ, USA) and then subcloned into the lentiviral plasmid backbone between the NhE1 site and the HincII site.
[0302] Lentiviral vectors encoding CAR constructs were generated by transient transfection of the 293T cell line. Briefly, 293T cells were seeded into poly-D-lysine-coated 15 cm plates (BD Biosciences, San Jose, CA, USA). The next day, plasmids encoding the CAR constructs together with packaging and envelope vectors (pMDLg / pRRE, pMD-2G, and pRSV-Rev) were transfected into 293T using lipofectamine 3000 (Life Technologies, Carlsbad, CA, USA). cell. Lentiviral supernatants were collected 48-72 hours after transfection, centrifuged at 3000 RPM for 10 minutes to r...
Embodiment 2
[0311] This example demonstrates surface expression on human T cells of a CAR cleaved from a CAR construct according to an embodiment of the invention compared to other CARs.
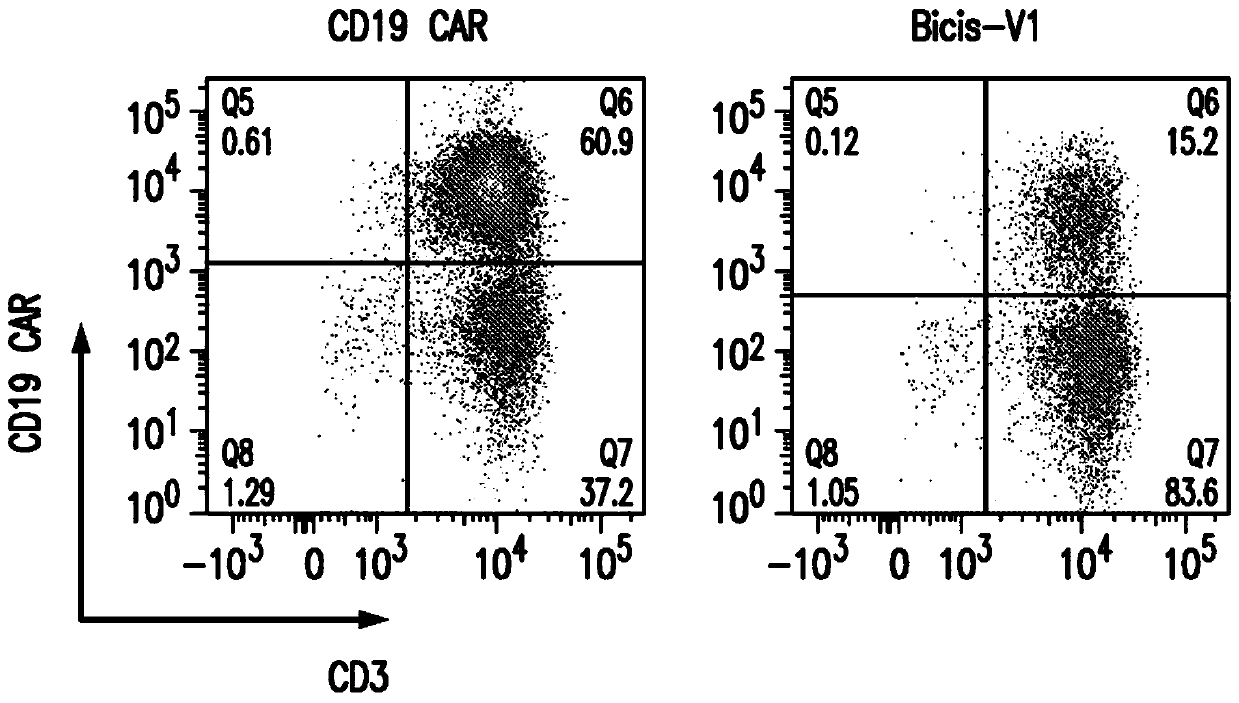
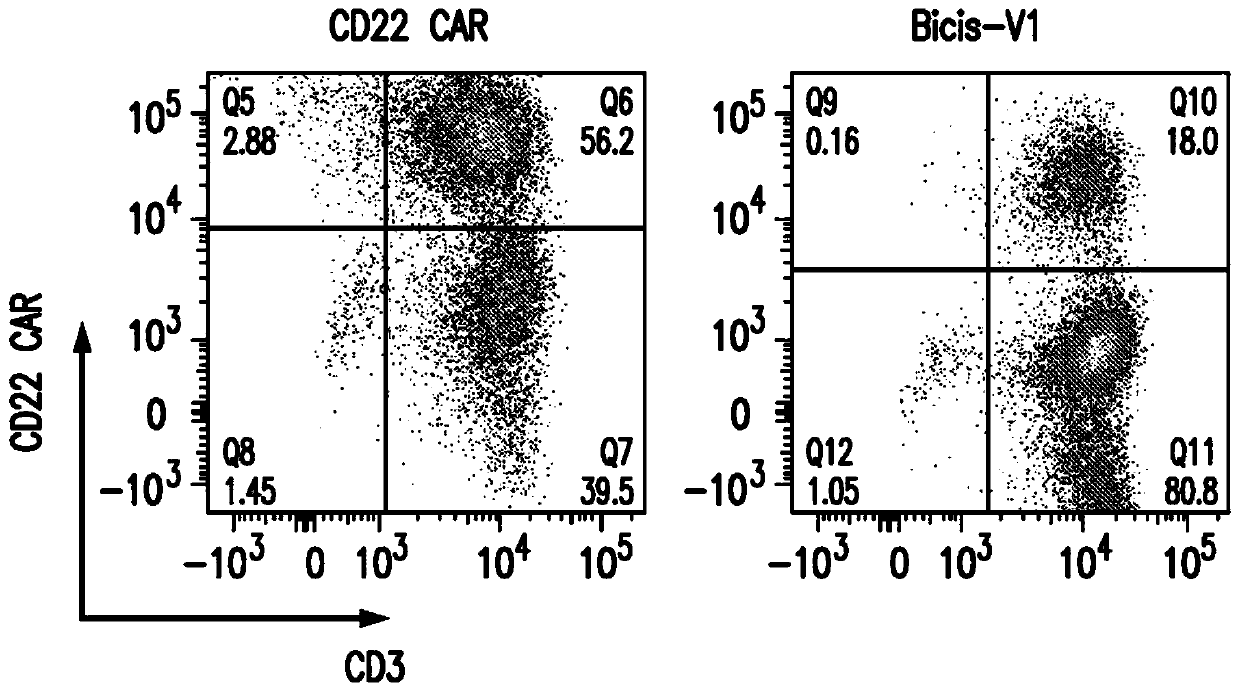
[0312] The surface expression of anti-CD19 CAR and anti-CD22 CAR on V1-transduced T cells was about 15%, while the expression of anti-CD19 CAR from vectors encoding only a single anti-CD19 CAR was 61%, and that from vectors encoding only a single anti-CD22 The expression of the anti-CD22 CAR of the CAR vector was 56% ( Figures 2A-2C ).
[0313] Human PBMC from healthy donors were activated for 24 hours with CD3 / CD28 microbeads. Activated T cells were then transduced with the vector alone or co-transduced with both a single anti-CD19 CAR and a single anti-CD22 CAR vector. Surface expression of anti-CD19 CAR and anti-CD22 CAR was analyzed on day 8. Co-transduced T cells had much lower expression of both anti-CD19 and anti-CD22 CARs compared to the bispecific cyclic CAR6. Anti-CD19 and anti-CD22 CARs ...
Embodiment 3
[0316] This example demonstrates the in vitro activity of CAR constructs according to embodiments of the present invention compared to other CARs based on cytokine production.
[0317] CAR-transduced T cells (1E5) were washed 3 times with 1×PBS, and then co-incubated with an equal amount of target cells in 200ml RPMI medium in a 96-well plate in a 37°C incubator for 15 to 20 hours. Target cells were K562 expressing CD19 or CD22 or both CD19 and CD22. K562 cells were used as a negative control. Cytokines levels of IL2 and IFNγ in culture supernatants were measured with ELISA kits (R&D Systems, Minneapolis, MN, USA). All tests were set up in triplicate. V1 CAR T cells produced large amounts of IL2 and IFNg when co-cultured with CD22-expressing target cells, but produced only low levels of IL2 and IFNg when co-cultured with CD19-expressing target cells ( Figure 5A and 5B ).
[0318] The CML cell line K562 was artificially transduced with CD19 or CD22 or both to express the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com