Polypeptide for inhibiting PD-L1 palmitoylation modification and expression and application of polypeptide
A technology of PD-L1 and palmitoylation, which is applied to polypeptides containing positioning/targeting motifs, chemical instruments and methods, peptides, etc., can solve the problems of reduced curative effect and poor effect, and achieve good inhibition principles and effects , the effect of reducing the content of PD-L1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 identifies the site and basic sequence of palmitoylation of endogenous PD-L1 expressed by tumor cells, and proves that palmitoylation inhibits the degradation of PD-L1 by lysosomes.
[0040] (1) Identify the site and basic sequence of palmitoylation of PD-L1:
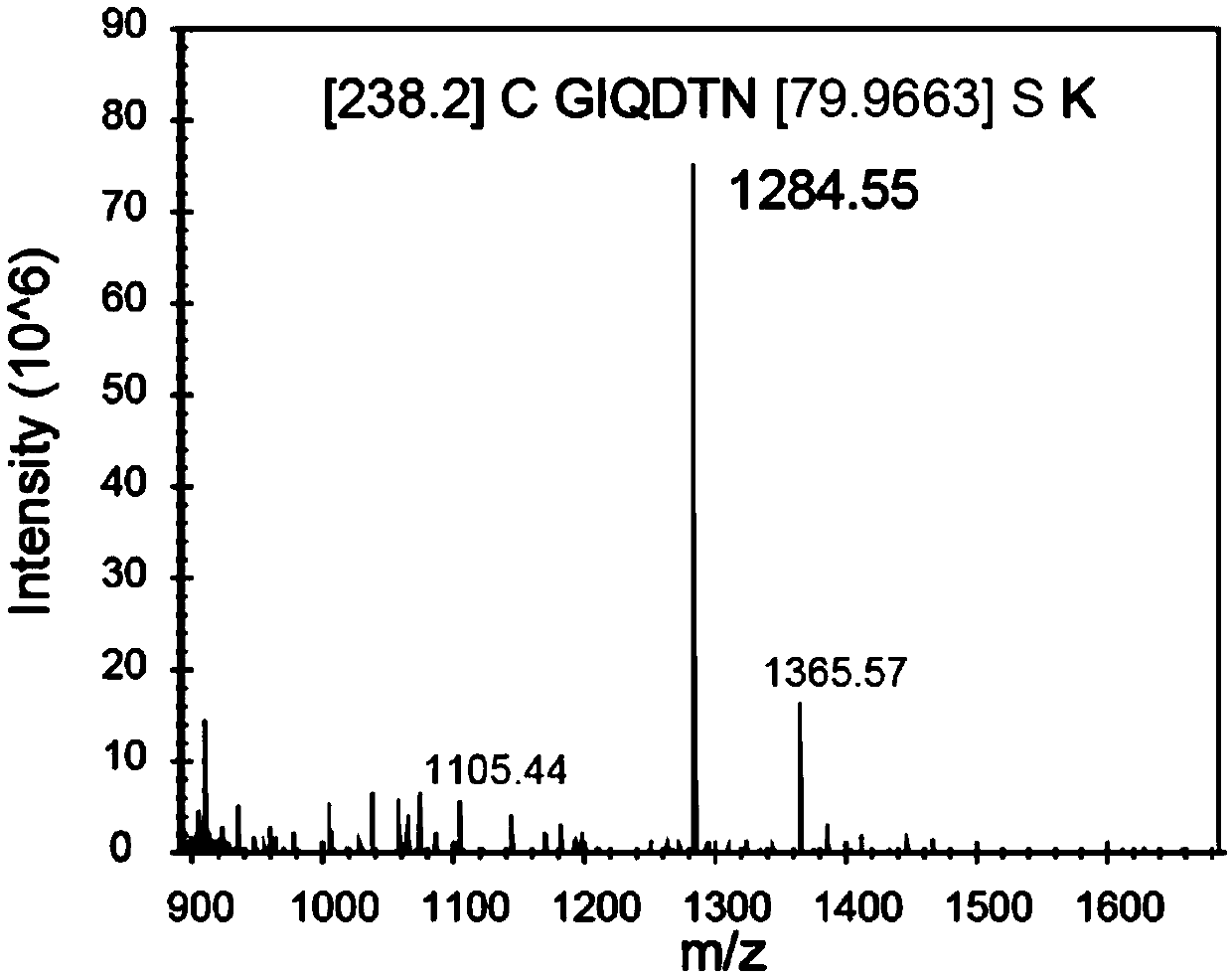
[0041] The endogenous PD-L1 expressed in HCT116 human colorectal cancer cells was purified by immunoprecipitation, gel electrophoresis was performed, and the Coomassie brilliant blue stained band corresponding to the molecular weight of PD-L1 was cut, digested with trypsin, and analyzed by liquid chromatography. The obtained peptides were analyzed by mass spectrometry and compared with the FindMod database to identify post-translational modifications of PD-L1. Such as image 3 As shown, the cysteine C272 at position 272 in the endogenous PD-L1 expressed by HCT116 cells undergoes palmitoylation.
[0042] (2) Proving that palmitoylation inhibits the lysosomal degradation of PD-L1.
[0043] In order t...
Embodiment 2
[0044] Example 2 Design of PD-L1 palmitoylation inhibitory polypeptide S1 (PD-palm), its localization and expression in cells, and its effect on PD-L1 expression.
[0045] (1) Using the principle of competitive inhibition to design PD-palm, a polypeptide inhibiting palmitoylation of PD-L1.
[0046] Given that the palmitoylation site of PD-L1 is C272, the short peptide motif modified by palmitoylation is usually determined by the common characteristics of 14 amino acids near the modification site. Therefore, the PD-L1 (265-279) short peptide was designed as a "bait" that can undergo palmitoylation modification, and the principle of competitive inhibition was used to reduce the palmitoylation modification of endogenous PD-L1.
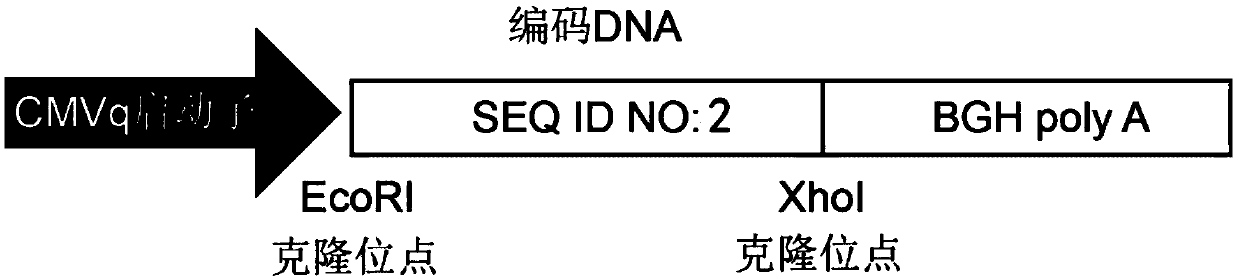
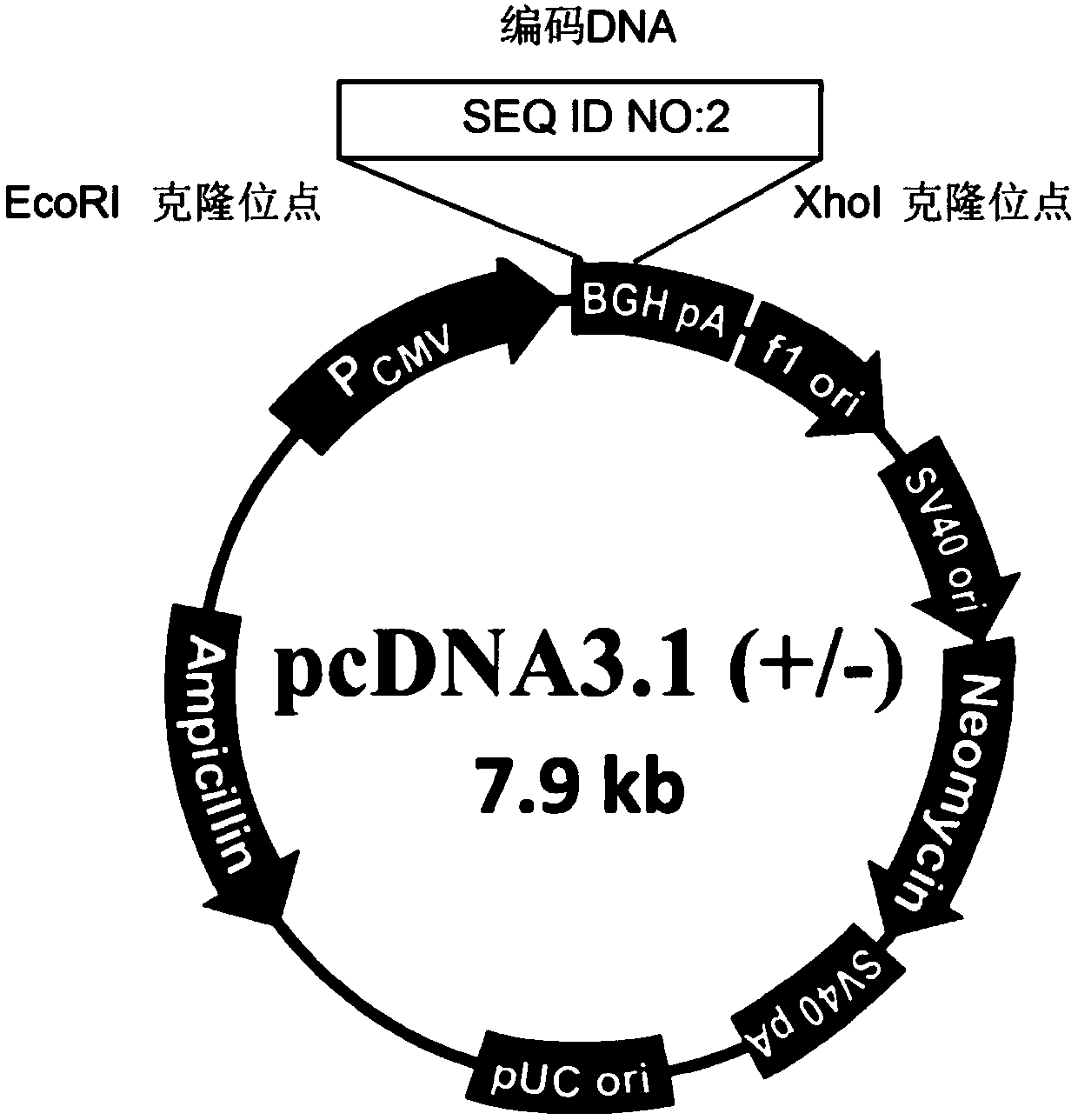
[0047] Such as figure 1 and figure 2 As shown, through the EcoRI and Xhol cloning sites, the nucleic acid sequence (SEQ ID NO: 2) encoding the polypeptide S1 (PD-palm) sequence (SEQ ID NO: 1) in the nucleic acid construct was inserted into the eukaryot...
Embodiment 3
[0051] Example 3 Using cell-penetrating peptides to transport PD-palm polypeptides synthesized in vitro into cancer cells to promote the degradation of PD-L1
[0052] The cell-penetrating peptide CPP can effectively carry polypeptides synthesized in vitro into cells, so if Figure 9 As shown, the fusion polypeptide CPP-S1 (PD-palm) of CPPtat and PD-palm and CPP-S2 as a control were constructed, wherein CPPtat is HIV-1 virus TAT protein transduction peptide. Synthesized by the standard Fmoc solid-phase synthesis method, after purification by high-performance liquid chromatography, CPP-S1 (PD-palm) was identified by mass spectrometry, and the identification results were as follows Figure 10 shown.
[0053] CPP-S1 (PD-palm) and CPP-S2 were added to HCT116 human colorectal cancer cell culture medium at different final concentrations (0, 1.2, 2.5, 5, 10 μM), incubated for 24 hours, and analyzed by immunoblotting Methods To detect the expression of PD-L1. Such as Figure 11 A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com