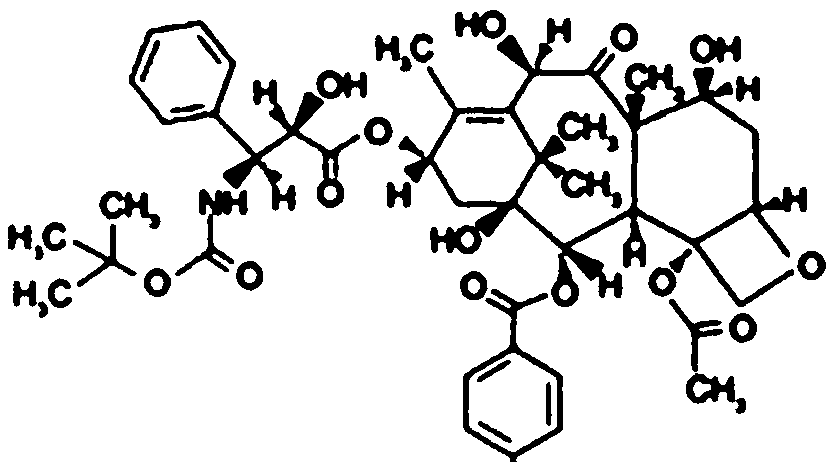
Docetaxel composition for injection and preparation method thereof
A technology of docetaxel and composition, applied in the field of docetaxel composition for injection and preparation thereof, can solve the problems of difficulty in realizing liquid form, difficulty in preparing docetaxel preparation, shortening of stabilization time, etc. , to achieve the effect of long stability time, long redissolving stability time and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
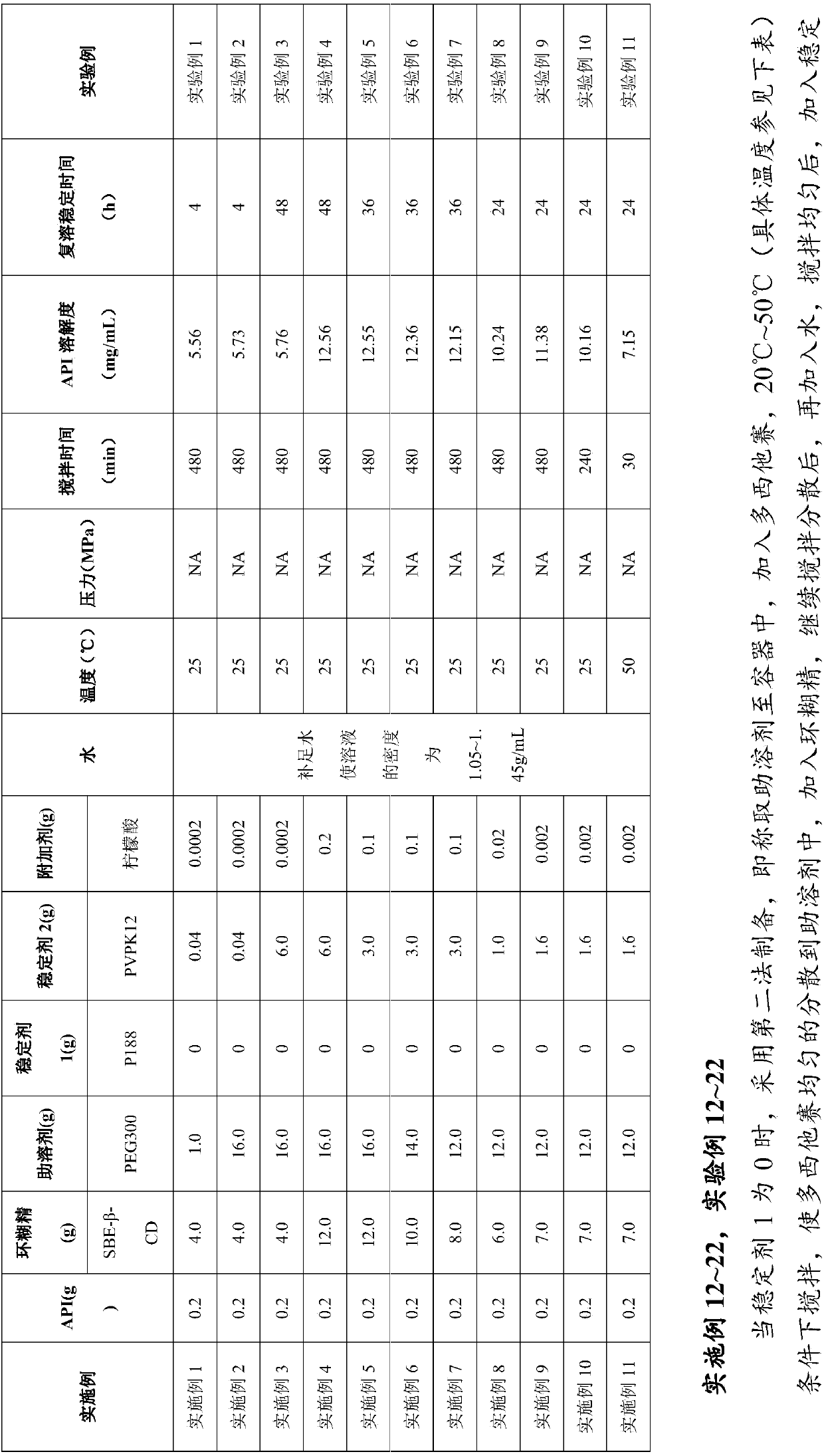
Embodiment 1~11
[0085] Embodiment 1~11, experiment example 1~11
[0086] When the stabilizer 1 is 0, use the first method to prepare, that is, weigh the cyclodextrin into the container, add the co-solvent and water, stir and dissolve it at 20°C~50°C (see the table below for the specific temperature), and then add Stir to dissolve stabilizer 1, stabilizer 2 and additives, then add docetaxel with or without nitrogen gas, stir and disperse until dissolved, then continue to stir for 30-480 minutes to obtain a uniform mixed solution; take samples to measure the pH value and Content, after passing the test, filter with a 0.2 μm microporous membrane, pack in vials, fill with nitrogen or not, press the stopper, roll the cap, and label it;
[0087]
[0088]
[0089]
[0090]
[0091]
[0092]
[0093]
[0094]
[0095]
[0096]
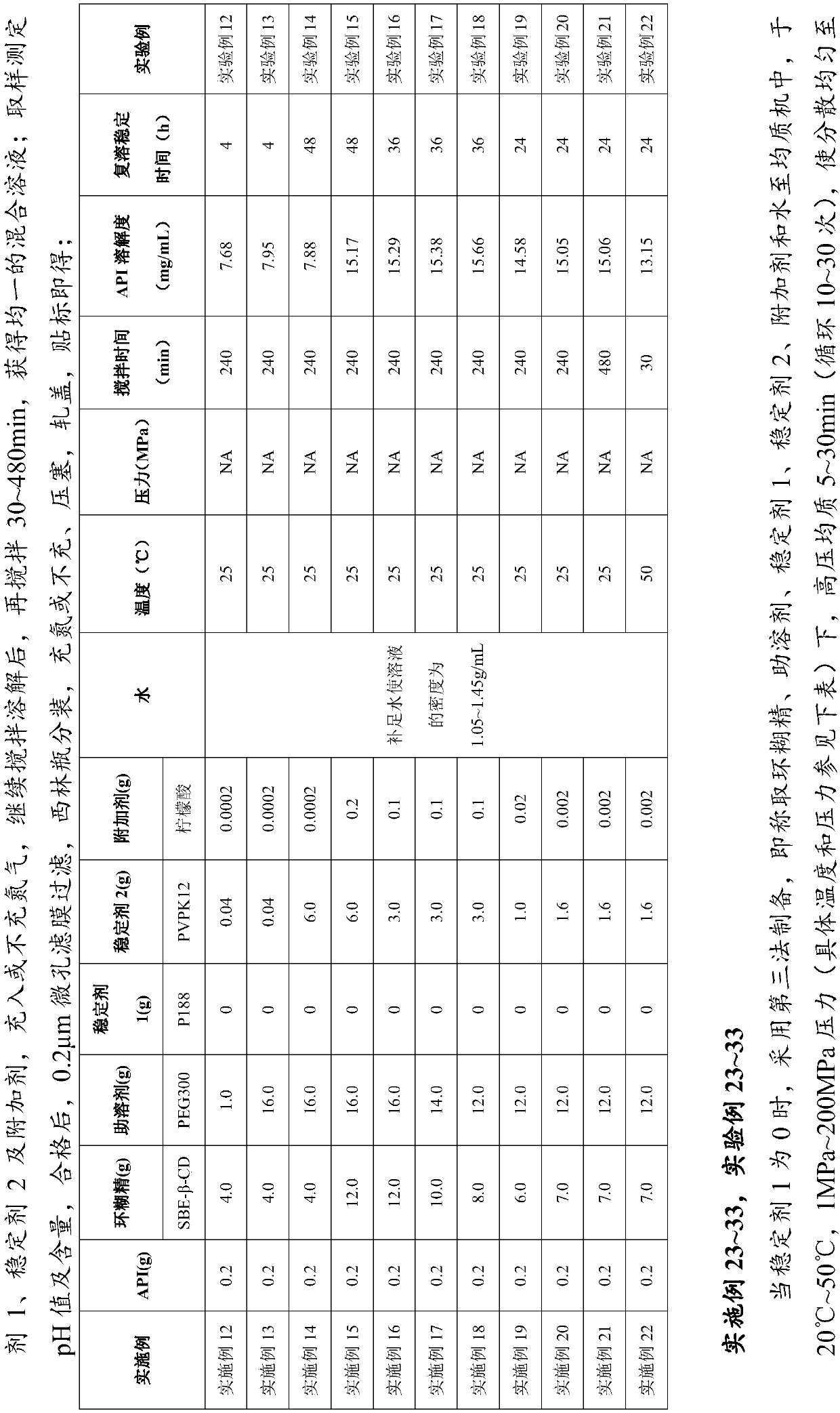
Embodiment 100
[0098]
[0099] Prepared by the fourth method, that is, weighed into a co-solvent homogenizer, added docetaxel, 25 ° C, 100 MPa, high-pressure homogenization for 5 minutes, so that the docetaxel was uniformly dispersed and suspended or dissolved in the co-solvent, Add cyclodextrin, stabilizer 1, stabilizer 2, additives, and water, and continue homogenizing for 30 minutes to obtain a homogeneous mixed solution; take a sample to measure the pH value and content, and filter it with a 0.2 μm microporous membrane and transfer it to a vial Subpackage, freeze-dry in a freeze dryer, fill with nitrogen, stopper, cap, and label.
[0100] Reconstitution stability time: 24h.
[0101] In order to further illustrate the innovation of the present invention, the inventor has done the following comparative examples and relevant experimental examples, specifically as follows:
experiment example 100
[0109] Determination of ethanol content: adopt gas chromatographic method to determine, specifically as follows:
[0110] Chromatographic conditions:
[0111] Chromatographic column: InertCap 624 (fixative solution is G43, Lenth: 60m; ID: 0.25mm, 1.4μm, Shimadzu):
[0112] Detector: hydrogen flame ionization detector (FID);
[0113] Carrier gas: nitrogen;
[0114] Linear speed: 21.5cm / s;
[0115] Total flow: 24.0mL / min;
[0116] Column flow: 1mL / min
[0117] Purge flow: 3.0mL / min;
[0118] Split ratio: 20:1.
[0119] Temperature programming conditions: initial temperature of 50°C, hold for 5 minutes, increase to 200°C at 10°C / min, hold for 4 minutes, and collect for a total of 24 minutes.
[0120] Injection port temperature: 210°C;
[0121] FID temperature: 280°C.
[0122] Injection volume: 1μL
[0123] Detection limit: 5.057ng
[0124] Limit of quantitation: 1.264ng
[0125] The samples of embodiment 9, embodiment 86 and comparative example 8 are respectively meas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com