A kind of vincristine compound impurity and its preparation method and application
A vincristine and compound technology, applied in organic chemistry, instruments, measuring devices, etc., can solve problems such as complex structure and preparation process, and impurities that are not fully understood and valued
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
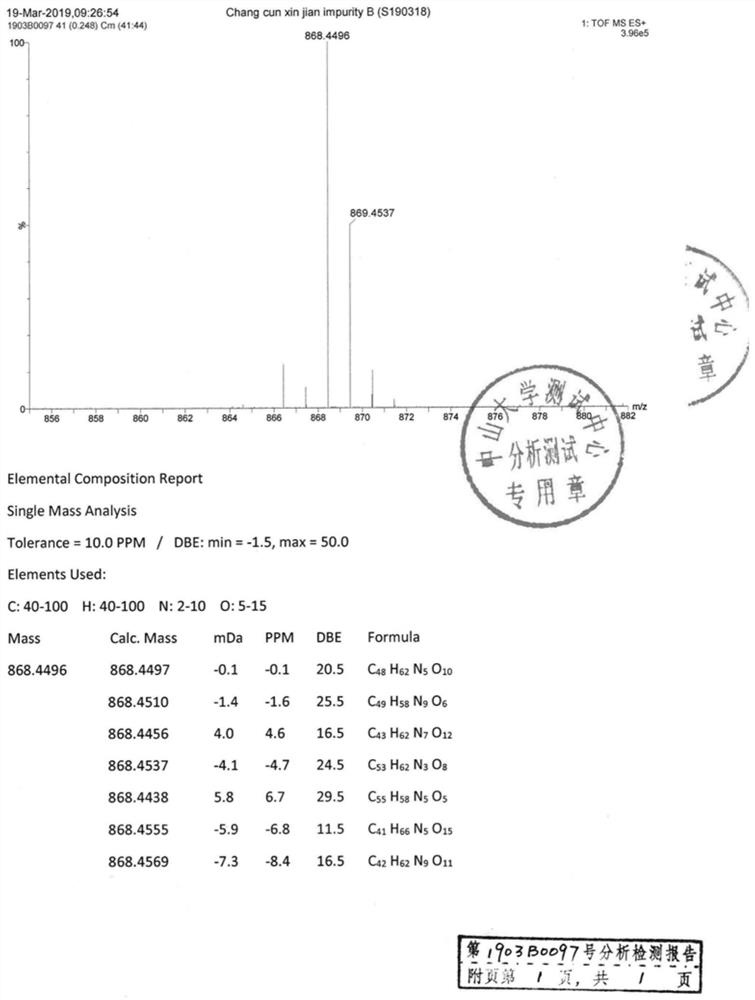
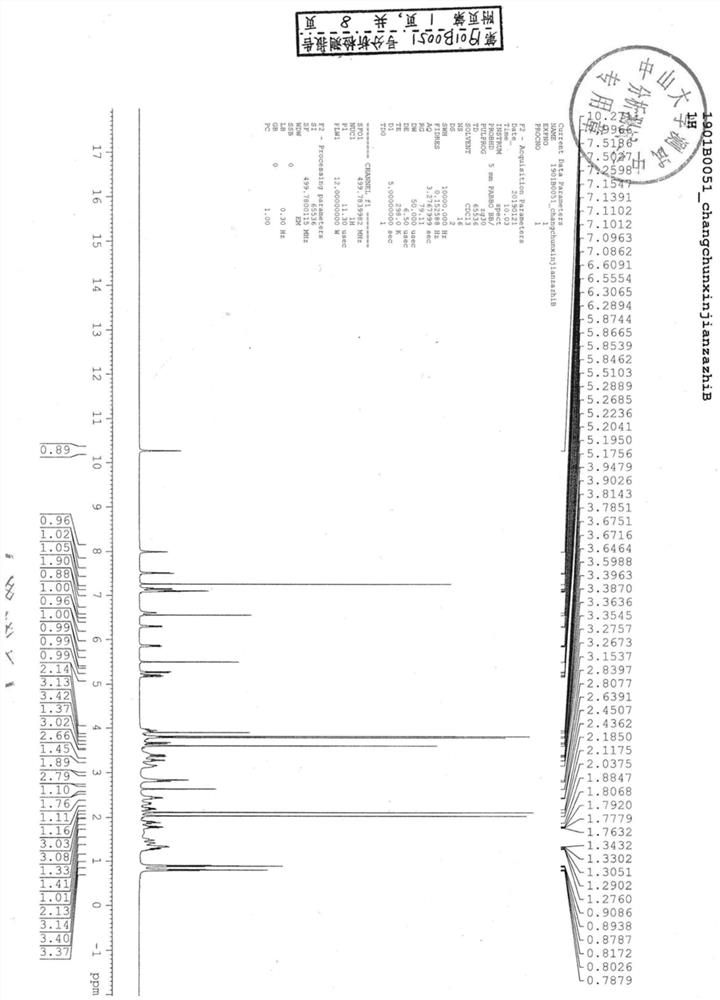
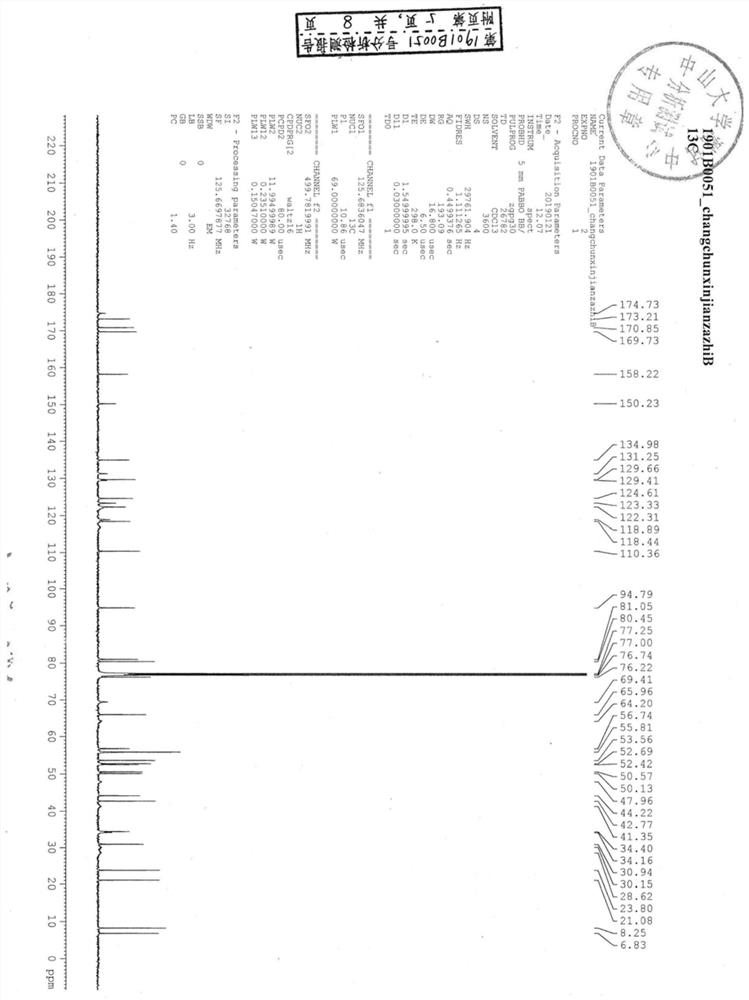
Image
Examples
Embodiment 1
[0046] A kind of its preparation method of vincristine compound impurity, comprises the following steps:
[0047] A. Preparation of crude vinblastine impurity product-I: Add 250 g of vinblastine total alkali sulfate, 3000 ml of acetone, 500 ml of acetic acid, and 1000 ml of acetic anhydride in a 30 L reaction tank, and stir. Add the mixed solution of 150ml of concentrated sulfuric acid, 200g of potassium dichromate and 15L of drinking water dropwise at -40°C. After the dropwise addition, the reaction was carried out for 1-2 hours, and the reaction of the raw material vinblastine was detected by TLC tracking (developing solvent, petroleum ether: chloroform: acetone: diethylamine=60:30:5:6). Stir slowly under an ice bath at -40°C, and slowly add 1500ml of concentrated ammonia water into the reaction tank dropwise. After mixing evenly, the pH of the solution is detected to be 8-9. 10L×5 trichloromethane extraction. The extracted chloroform solution was washed three times with 3...
Embodiment 2
[0053] A preparation method for vincristine compound impurities, comprising the following steps:
[0054] A. Preparation of crude vinblastine impurity product-I: Add 250 g of vinblastine total alkali sulfate, 2250 ml of acetone, 375 ml of acetic acid, and 750 ml of acetic anhydride in a 30 L reaction tank, and stir. A mixed solution of 125ml of concentrated sulfuric acid, 125g of potassium dichromate and 12.5L of drinking water was added dropwise at -42°C. After the dropwise addition, the reaction was carried out for 1-2 hours, and the reaction of the raw material vinblastine was detected by TLC tracking (developing solvent, petroleum ether: chloroform: acetone: diethylamine=60:30:5:6). Keep stirring slowly under the ice bath at -42°C, and slowly add 1500ml of concentrated ammonia water into the reaction tank dropwise. After mixing evenly, the pH of the solution is detected to be 8-9. 10L×5 trichloromethane extraction. The extracted chloroform solution was washed three times...
Embodiment 3
[0059] A preparation method for vincristine compound impurities, comprising the following steps:
[0060] A. Preparation of crude vinblastine impurity product-I: Add 750 g of vinblastine total alkali sulfate in a 100 L reaction tank, add 10.5 L of acetone, 1875 ml of acetic acid, and 4500 ml of acetic anhydride, and stir. Add the mixed solution of 600ml of concentrated sulfuric acid, 750g of potassium dichromate and 75L of drinking water dropwise at -45°C. After the dropwise addition, the reaction was carried out for 1-2 hours, and the reaction of the raw material vinblastine was detected by TLC tracking (developing solvent, petroleum ether: chloroform: acetone: diethylamine=60:30:5:6). Keep stirring slowly under the ice bath at -45°C, and slowly add 4500ml of concentrated ammonia water into the reaction tank dropwise. After mixing evenly, the pH of the solution is detected to be 8-9. 30L×5 trichloromethane extraction. The extracted chloroform solution was washed three times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com