Impurities of heterocyclic boronic acid compounds and their control methods
A quality, tartrate technology, applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the difficulty of separation and identification, the large number of impurities in dudagliptin, and the difficulty of separation and identification of impurities, etc. problems, to achieve the effect of ensuring quality stability and drug safety, ensuring purity, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 impurity (II)
[0063]
[0064] Add 2 g of duagliptin L-tartrate and 100 mL of 0.1% hydrogen peroxide solution into the reaction bottle, and place it at room temperature for at least 90 h. The sample was lyophilized at -18°C to yield 1 g of impurity (II).
[0065] The impurity (II) is dissociated under alkaline conditions, and the L-tartaric acid therein is removed to obtain the impurity (I). During detection, impurity (I) and impurity (II) peak simultaneously.
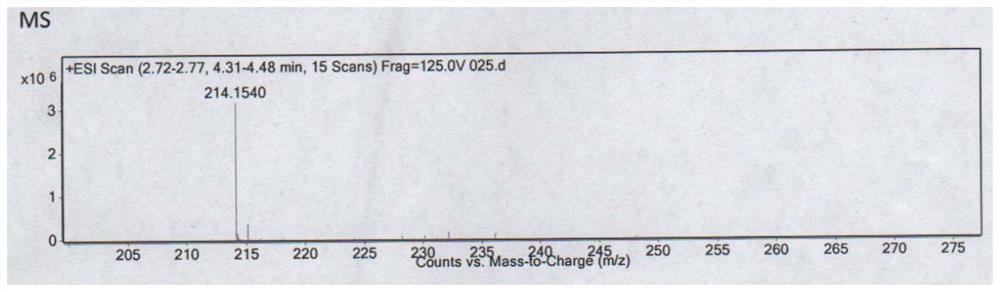
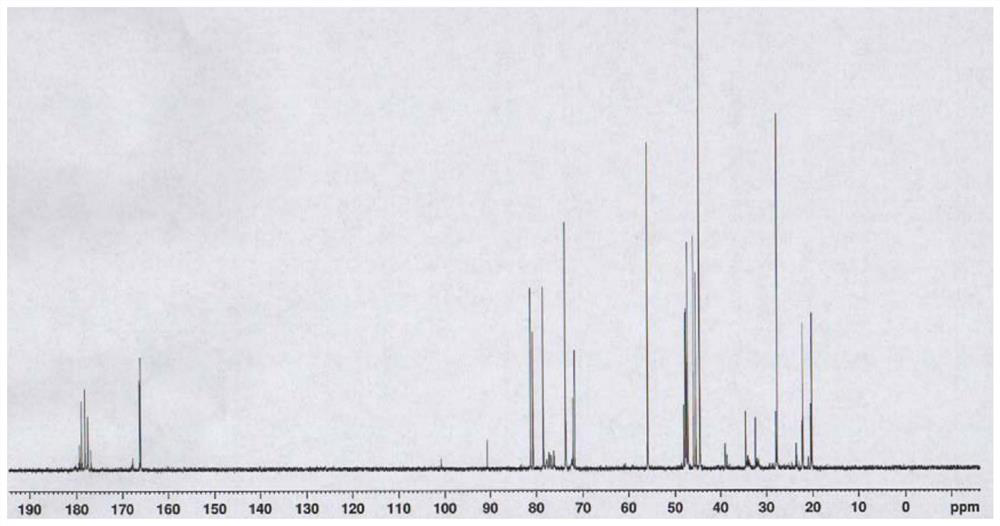
[0066] Gained impurity (II) is carried out structural identification, obtains following data:
[0067] MS(ESI)m / z=214.1540(M+H) + , consistent with the molecular weight of impurity (II) free base 213.1477. Spectrum see attached figure 1 .
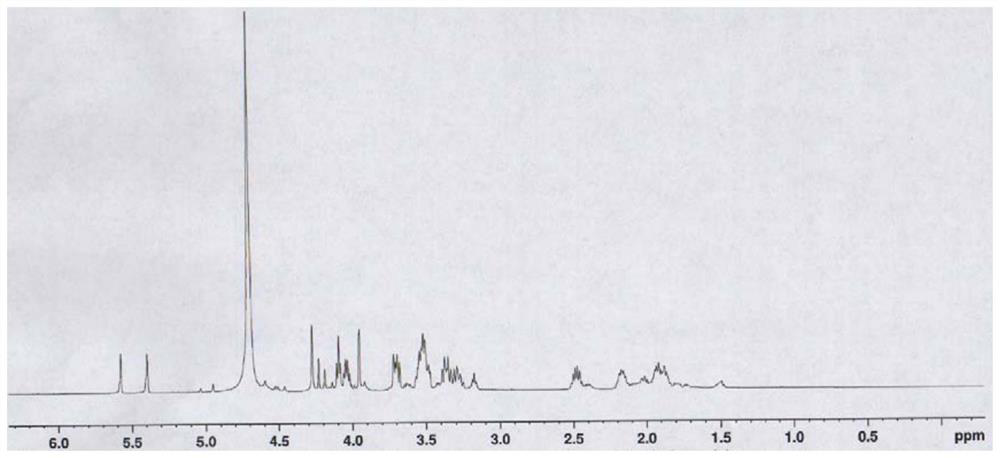
[0068] 1 H NMR (500MHz, D 2 O) δ5.75(s, 1H), 5.39(s, 1H), 4.27(s, 2H), 4.10-4.08(m, 1H), 4.06-4.02(m, 1H), 3.95(s, 1H), 3.72-3.68(m, 1H), 3.55-3.50(m, 3H), 3.38-3.27(m, 2H), 2.51-2.44(m, 1H), 2.18-2.14(m, 1H), 1.94-1.86(m , 3H), s...
Embodiment 2
[0071] The preparation of embodiment 2 high-purity Gliptin L-tartrate
[0072] 1), hydrogenation reaction
[0073]
[0074] Intermediate (2-I) (5.7Kg), 5% wet Pd / C (0.57Kg) and methanol (43L) were added to the reaction kettle. The air in the system was replaced three times with nitrogen, and then three times with hydrogen. Under the hydrogen pressure of 0.5MPa, react at room temperature, when the system pressure drops to 0.3MPa, add hydrogen to the pressure of 0.5MPa, replace the reaction system with hydrogen twice every hour, and react for 5h.
[0075] Fill the pressure filter with microcrystalline cellulose and anhydrous sodium sulfate, pressurize the reaction solution with nitrogen, rinse with methanol, combine the filtrates, concentrate under reduced pressure under nitrogen protection to a residual volume of about 10L, add 14L of ethyl acetate At this time, the mixture is a milky cloudy liquid, continue to concentrate to a remaining volume of about 10L, add 14L of eth...
Embodiment 3
[0080] Example 3 Large-scale preparation of high-purity Gliptin L-tartrate
[0081] 1), hydrogenation reaction
[0082]
[0083] Add intermediate (2-I) (70kg), 5% wet Pd / C (7Kg) and methanol (500L) into the reaction kettle. Replace the air in the system with nitrogen three times, then replace it with 0.5MPa hydrogen three times, stir the reaction at room temperature, add hydrogen to the pressure of 0.5MPa when the system pressure drops to 0.3MPa, replace the reaction system with hydrogen twice every hour, and react for 7h , to terminate the reaction.
[0084] Fill the pressure filter with microcrystalline cellulose and anhydrous sodium sulfate, filter the reaction liquid under pressure with nitrogen, and rinse with methanol. The filtrates were combined, concentrated under reduced pressure under nitrogen protection, and ethyl acetate (180 L) was added. At this time, the mixture was a milky turbid liquid, and the concentration was continued to a residual volume of about 140...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com