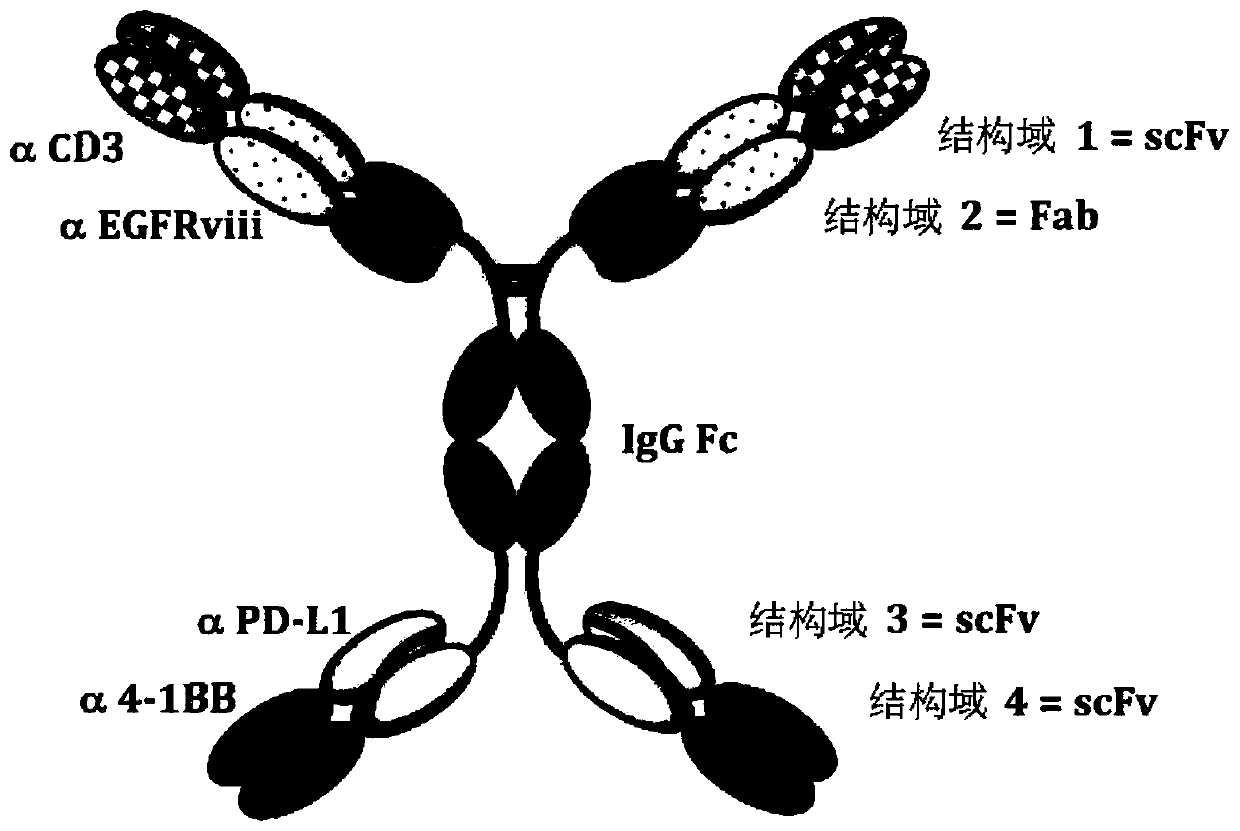
Multi-specific antibodies and methods of making and using thereof
A specific and binding specific technology, applied in chemical instruments and methods, antibodies, specific peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1) the binding of tetraspecific antibody and EGFRvIII antigen
[0106] Binding of the tetraspecific antibodies listed in Table 1 to the EGFRvIII antigen expressed on the surface of the U87 cell line was assessed using the FACS method. Tetraspecific antibodies were incubated with the U87 cell line and then detected with a secondary anti-human antibody directly conjugated to the Alexa Fluor647 fluorescent dye. Cellular binding of tetraspecific antibodies was analyzed on a flow cytometer BD LSRFortessa. All antibodies tested bound the antigen with KD in the single digit and subnanomolar range (Table 2). The observed differences in binding were in the 3-fold range and were likely driven by the position of the binding domain within the molecule as well as interactions with neighboring domains.
[0107] Table 1 shows examples of tetraspecific antibodies with EGFRvIII tumor antigen binding domains. Table 2 shows binding to EGFRvIII antigen expressed in the U87 c...
Embodiment 2
[0113] Example 2): Binding of tetraspecific antibodies to EGFRvIII, 4-1BB, PD-L1 and CD3 protein antigens
[0114] The binding affinities and kinetics of the tetraspecific antibodies listed in Table 1 to their respective antigens were evaluated by surface plasmon resonance on a Fortebio Octet RED96 instrument. Antigens are immobilized on the surface of the sensor chip, and the antibodies to be tested flow over the immobilized antigens. All molecules showed high binding to antigen (Table 3). SI-39E29, SI-39E18 and SI-39E23 showed lower binding to CD3 e / d antigen than other antibodies tested. Table 3 shows the binding of the four specific antibodies listed in Table 1 to EGFRvIII, 4-1BB, PD-L1 and CD3 antigens.
[0115] Table 3. Binding to EGFRvIII, 4-1BB, PD-L1 and CD3 antigens.
[0116] Table 3A.
[0117]
[0118] Table 3B.
[0119]
Embodiment 3
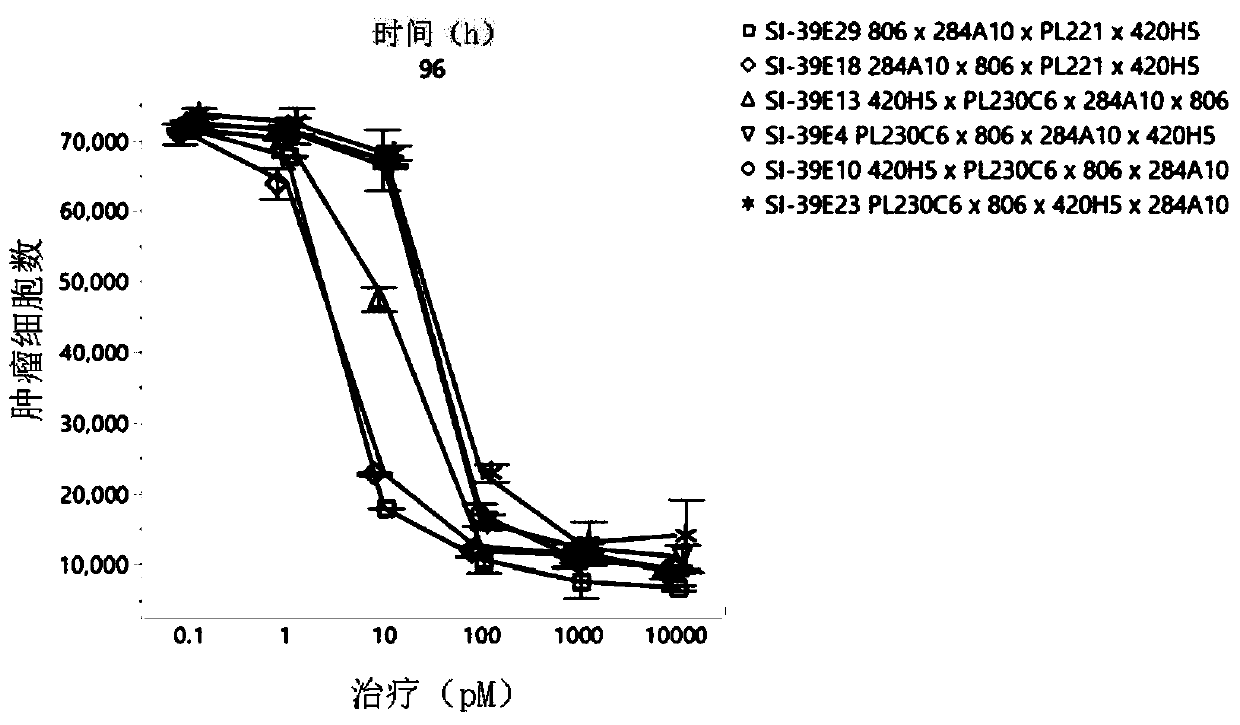
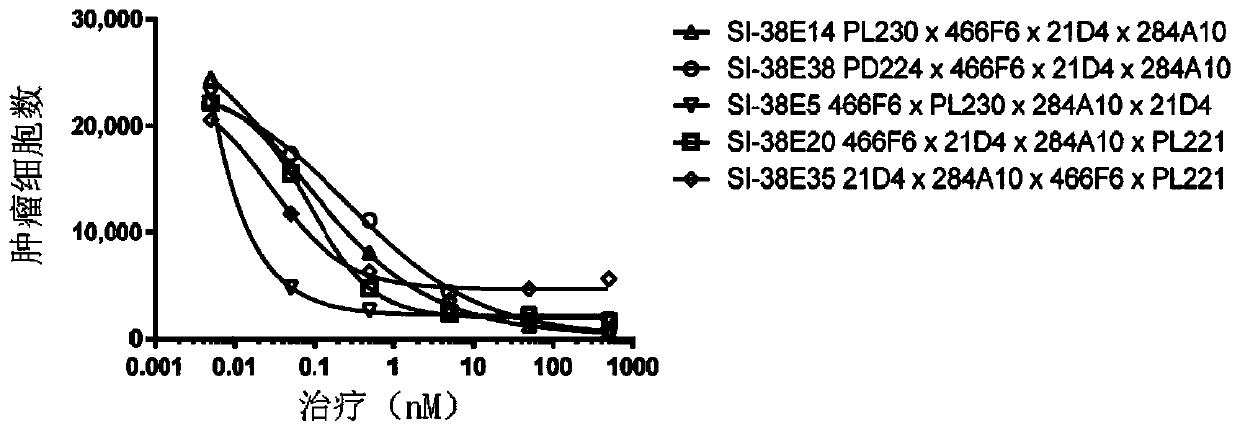
[0120] Example 3) Redirected PBMC cytotoxicity against the astrocytoma cell line U87 transfected with EGFRvIII
[0121] The tetraspecific antibodies listed in Table 1 were evaluated for their ability to redirect PBMCs to lyse U87 transfected with the EGFRvIII tumor cell line (U87vIII). PBMCs were separated by Ficoll gradient. The U87vIII tumor cell line stably expresses nuclear-localized red fluorescent protein (RFP) delivered by lentiviral transduction (Sartorius). U87vIII tumor cells were co-cultured with PBMCs. Tumor cell lysis was assessed by counting RFP-labeled tumor cell nuclei. Images were acquired on a live cell imager IncuCyte (Sartorius). SI-39E18 and SI-39E13 tetraspecific antibodies showed the highest potency at 96 hours, followed by SI-39E10. SI-39E4, SI-39E23 and SI-39E29 showed lower potency in this study than the other antibodies listed in Table 1 (Figure 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com