Application of lysophosphatidylethanolamine 18: 1 in preparing drug for relieving and treating inflammatory bowel diseases
A technology of phosphatidylethanolamine and inflammatory bowel disease, which is applied in the field of medicine and can solve problems such as unclear etiology of inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
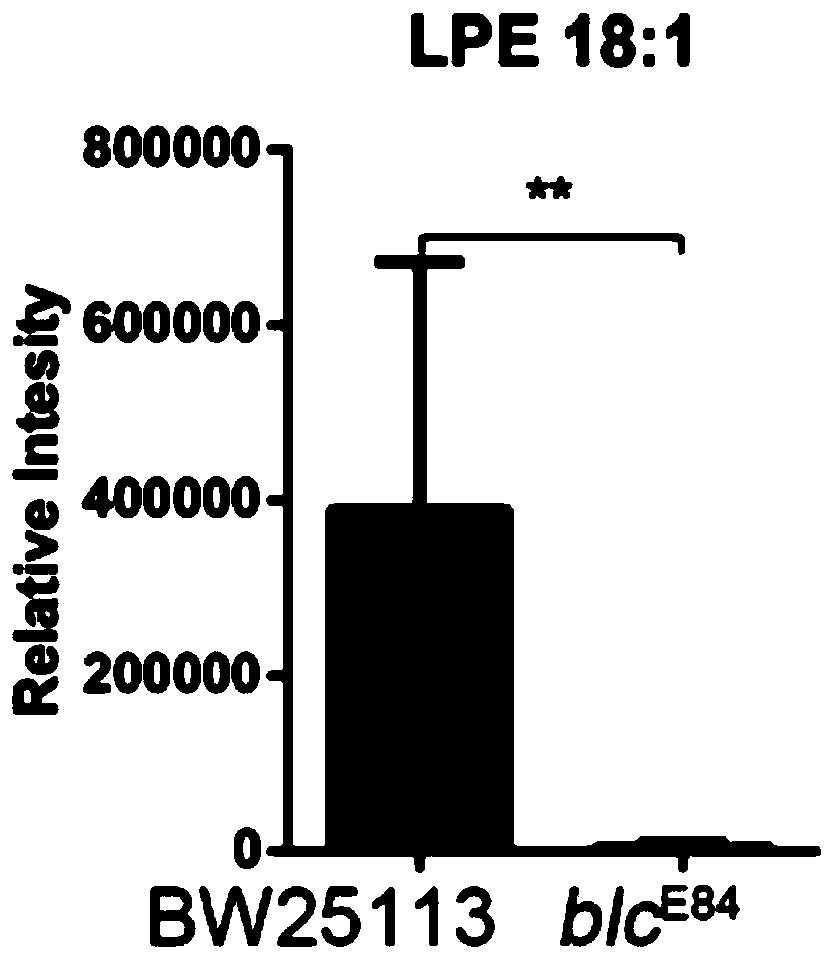
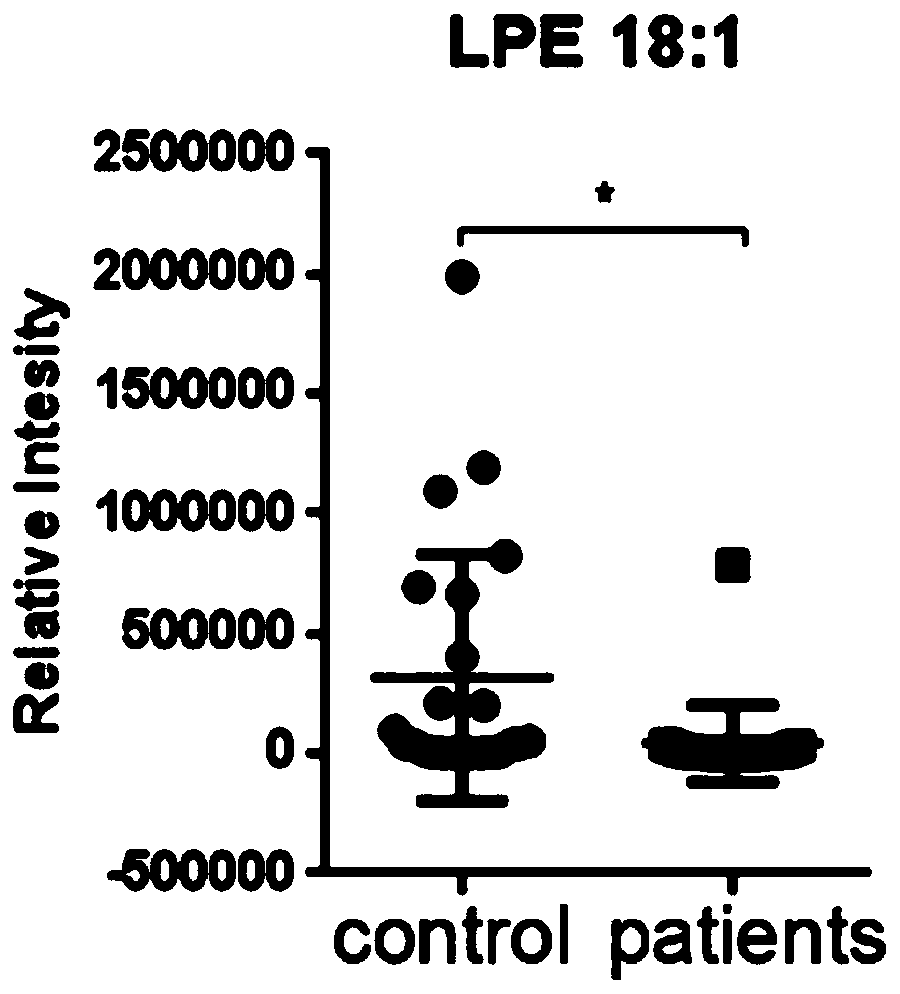
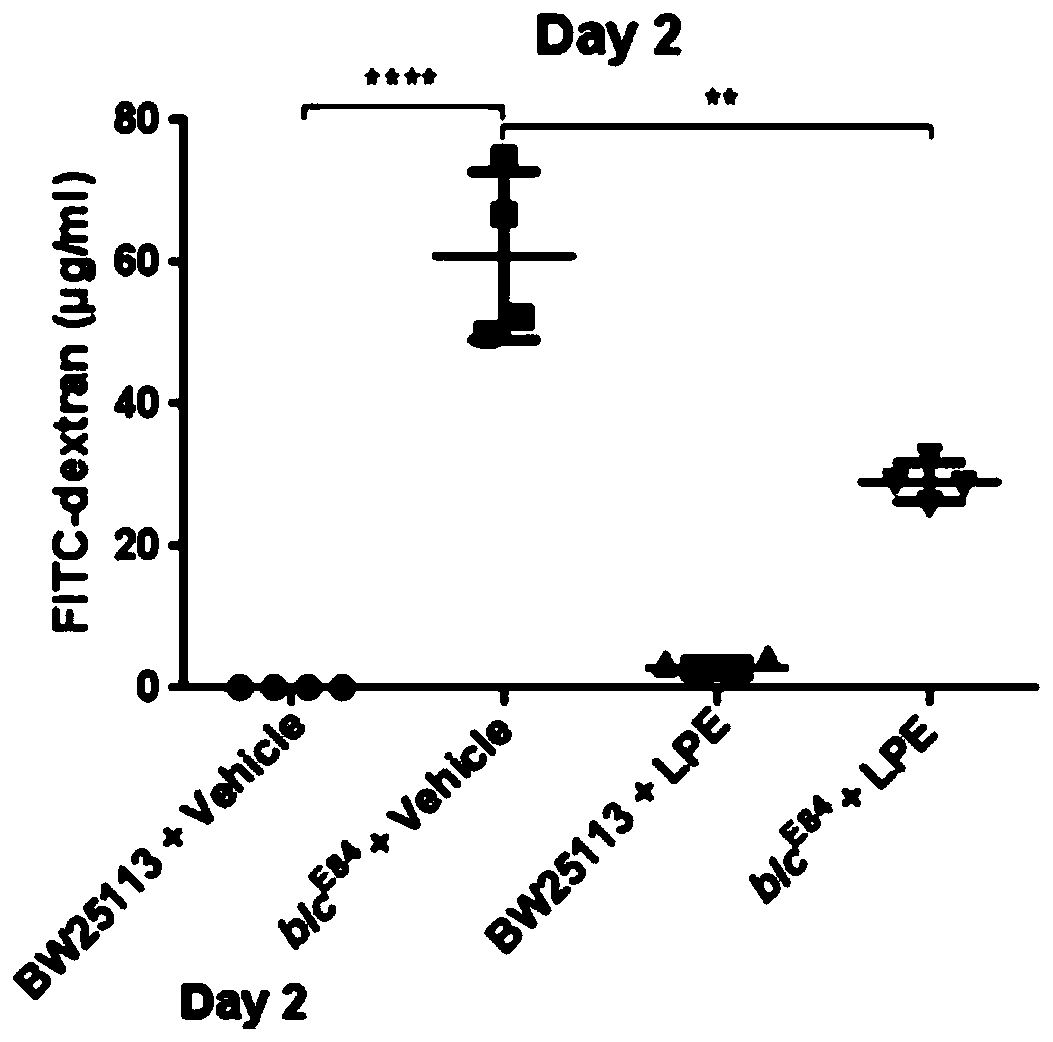
[0022] Experiment 1: Pathogenic Escherichia coli infection mouse model, detection of lysophosphatidylethanolamine 18:1 content in feces of enteritis mice and patients
[0023] IExperimental material
[0024] As experimental animals, 6-week-old male C57BL / 6N mice, weighing 17±2 grams, were selected from the Institute of Model Animals, Nanjing University.
[0025] II Test Method and Results
[0026] 2.1 Animal experiments
[0027] (1). Transfer 16 C57BL / 6N mice into the biological isolation chamber;
[0028] (2). Oral administration of antibiotic mixed solution (Ampicillin 5mg per mouse, Neomycin 5mg per mouse, Vancomycin 5mg per mouse, Metronidazole 4mg per mouse), for one week continuously;
[0029] (3). Change drinking water to mixed antibiotic water for one week (Ampicillin 1g per liter, Neomycin 1g per liter, Vancomycin 0.5g per liter, Metronidazole 1g per liter);
[0030] (4). Replace with normal drinking water;
[0031] (5). The mice were randomly divided into 4 grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com