Novel antitumor and immunosuppression compounds
A technology of compounds and hydrates, applied in the field of medicine, to achieve good industrial value or medicinal value, improve operability, and facilitate storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
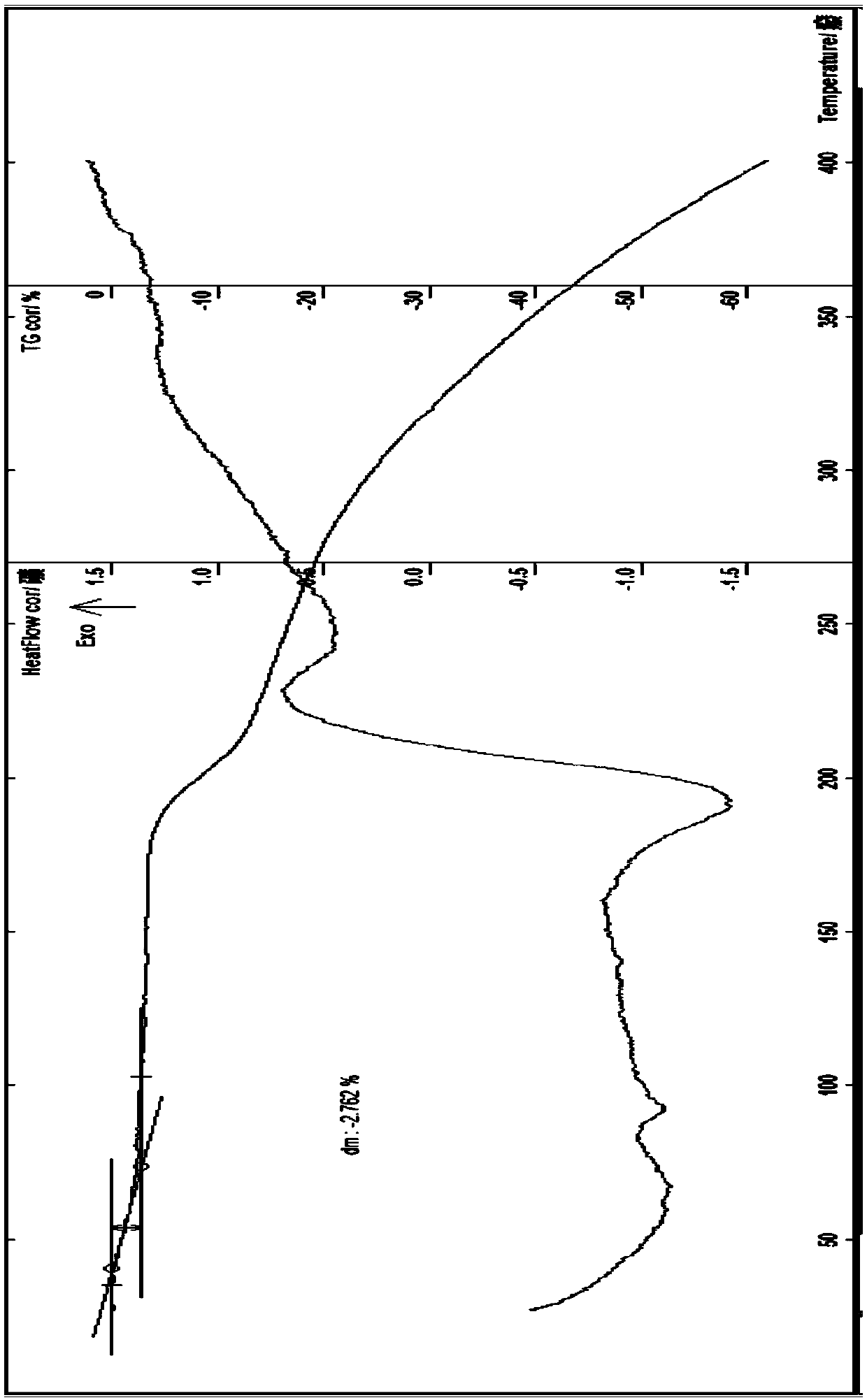
[0087] Embodiment 1 Preparation of everolimus 1.5 hydrate (ω-type compound)
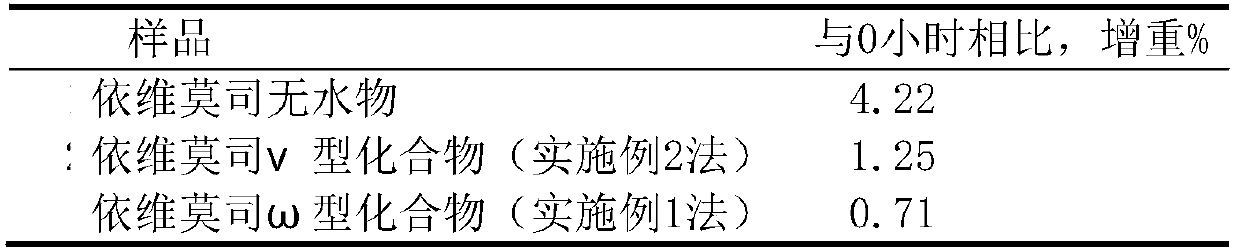
[0088] Add 10g of amorphous everolimus and about 30ml of isopropanol to a 250ml three-necked flask, protect it with nitrogen, stir, raise the temperature to about 40-60°C, add an appropriate amount of ethanol, stir until it is completely dissolved, and then add 0.05 M lactic acid 1ml, stir, add 60ml of 10% alcohol aqueous solution, cool to below 5°C and place, wait until the precipitate is fully separated, filter with suction, wash the solid with a small amount of cold water 3 times, filter with suction, dilute the solid in an oven at 28°C Air-dried for about 1 hour, and air-dried in an oven at 39°C for about 4 hours to obtain 8.6 g of off-white crystalline solid; identification: HPLC: the retention time of the main peak of the HPLC was the same as that of the everolimus anhydrous reference substance Consistent; Moisture measured by Karl Fischer method is 2.72%, thermal analysis: platform weight loss...
Embodiment 2
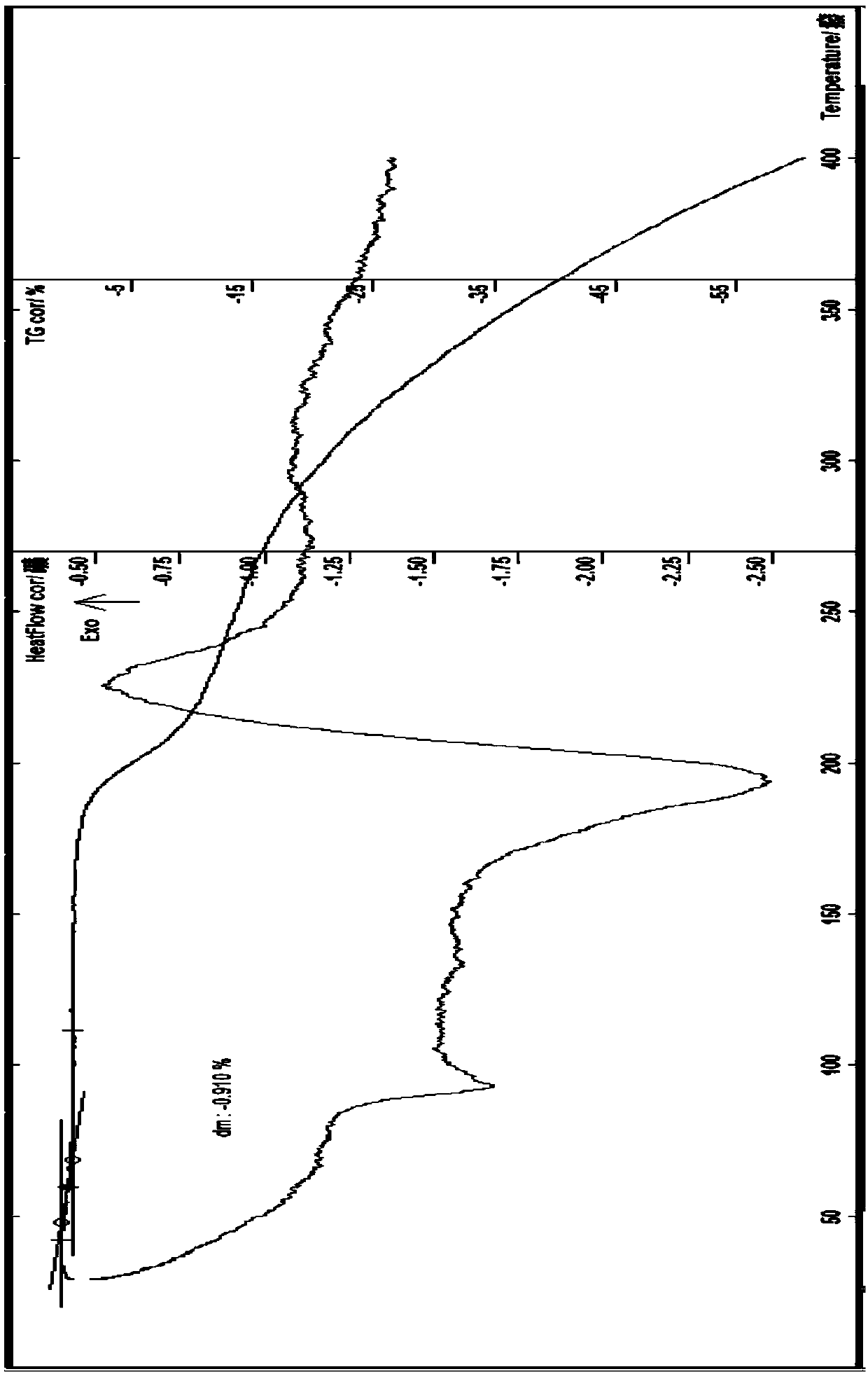
[0089] Embodiment 2 Preparation of everolimus 0.5 hydrate (v type)
[0090]Add 10g of amorphous everolimus and 40ml of absolute ethanol to a 250ml three-necked flask, protect it with nitrogen, stir, and add an appropriate amount of acetonitrile at about 40-50°C, stir until it is completely dissolved, and then add 0.02M lactic acid 1ml, stir, add 70ml of 10% ethanol aqueous solution, cool to below 0°C, and place until the precipitate is fully separated, filter with suction, wash once with a small amount of petroleum ether, wash 3 times with a small amount of ice-cold water, filter with suction, and dilute the obtained solid in Blast drying in an oven at about 50°C for 2 hours to obtain 7.8 g of off-white crystalline solid; identification: HPLC: HPLC main peak retention time is consistent with that of everolimus anhydrous reference substance HPLC main peak retention time; Karl Fischer method to determine moisture It is 0.89%; thermal analysis: about 0.91% of platform weight loss...
Embodiment 3
[0091] Embodiment 3 Preparation of new everolimus compound small-volume injection
[0092] Prescription: everolimus crystalline hydrate (prepared according to the method of Example 1, the main drug weight is calculated as everolimus) 1.0g, ethanol 20ml, ethylene glycol 10ml, mannitol 10g, Tween-80 2g, calcium EDTA Sodium 4 hydrate 0.1g, 1M lactic acid and sodium hydroxide solution, appropriate amount, water for injection appropriate amount to 1000ml;
[0093] Add 800ml of water for injection to the everolimus crystalline hydrate of the prescribed amount (prepared by the method of Example 1, and the weight is based on everolimus), ethanol, ethylene glycol, mannitol, Tween-80, EDTA calcium sodium , stir to dissolve completely, adjust the pH value within the range of 6.0-7.5 with an appropriate amount of lactic acid and sodium hydroxide solution, add water for injection to 1000ml, filter through an ultrafiltration membrane with a molecular weight cut-off of 10000-40000, and fill ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com