Humanized cxcr3 antibodies with depleting activity and methods of use thereof
A humanization, antibody technology, applied in chemical instruments and methods, medical preparations containing active ingredients, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0300] Example 1: Generation of Humanized Anti-CXCR3 Monoclonal Antibody
[0301] Anti-CXCR3 monoclonal antibodies were generated as described in WO2013 / 109974. Humanized clone 53 monoclonal antibody was generated as described below.
[0302] A humanized version of clone 53 capable of directing depletion of CXCR3 expressing cells was prepared. A humanized clone 53 monoclonal antibody with the VH and VK sequences shown in Table 4 was generated.
[0303] Germinal Index scores for each humanized variant shown in Table 4. The heavy chain was compared to the IGHV3-23*03 / IGHJ4*03 germline sequence; the light chain was compared to the IGKV1-9*01 / IGKJ2*01 germline sequence.
[0304] Table 4
[0305] Hu antibody VH SEQ ID NO VL SEQ ID NO Hair Growth Index 1 a
Hair Growth Index 2 b
23 18 21 0.885 0.950 24 19 21 0.872 0.933 25 20 21 0.877 0.939 26 18 22 0.890 0.955 27 19 22 0.877 0.939 29 20 22 0.881 0.944 ...
Embodiment 2
[0321] Example 2: CDR optimization
[0322] Many VH CDR and / or VL CDR variants were prepared. Binding affinity to recombinant human CXCR3 was measured using Biacore. Mutants with at least as strong binding as 53Hu37 are shown in Table 3.
Embodiment 3
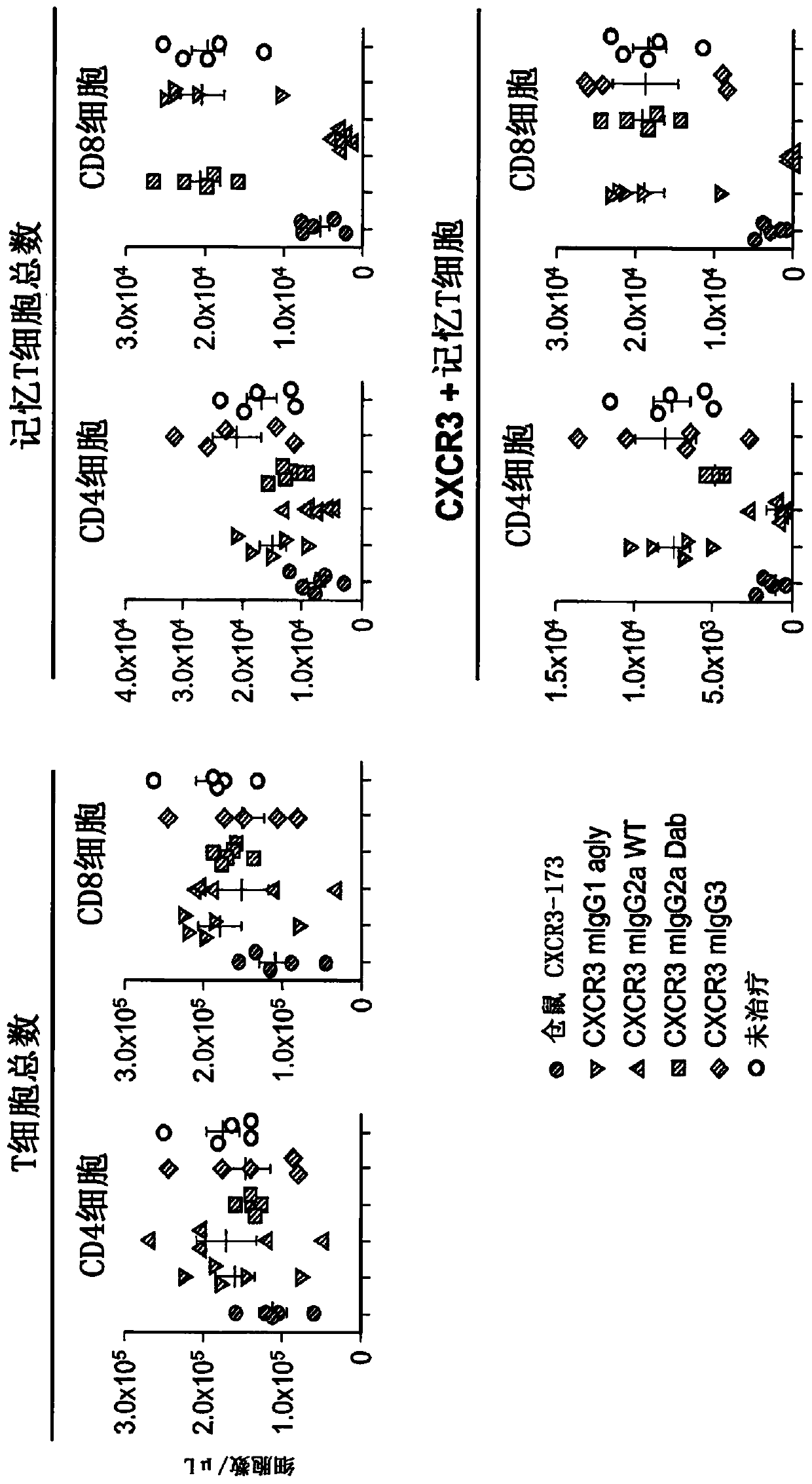
[0323] Example 3: CXCR3-173 is a depleting antibody
[0324] Hamster anti-mouse CXCR3 monoclonal (clone CXCR3-173) was used as a surrogate antibody in preclinical experiments. CXCR3-173 has been previously described as a blocking antibody that does not deplete CD4+ T cells in vivo. (See Uppaluri et al., Transplantation 86:137-47 (2008)).
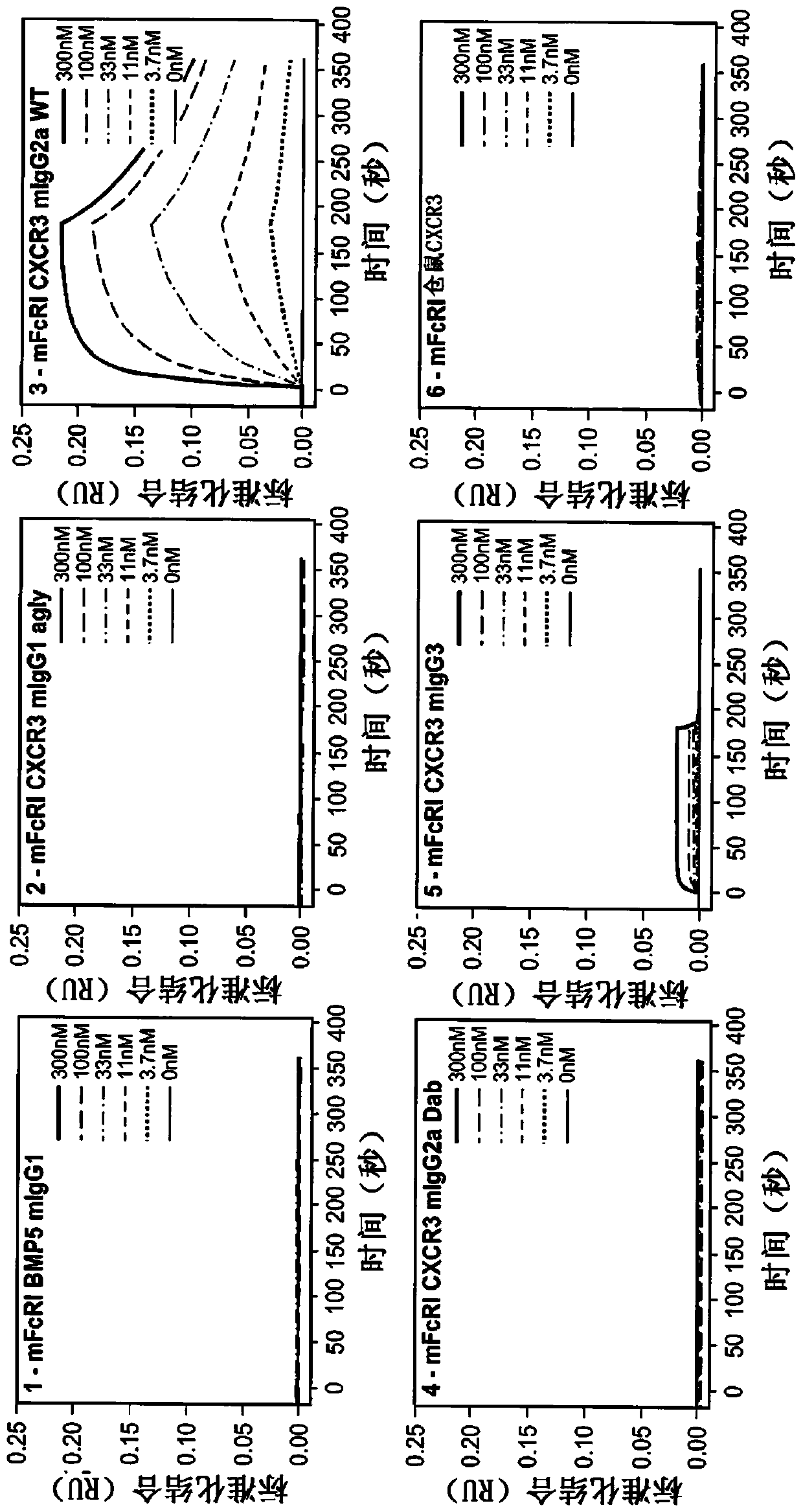
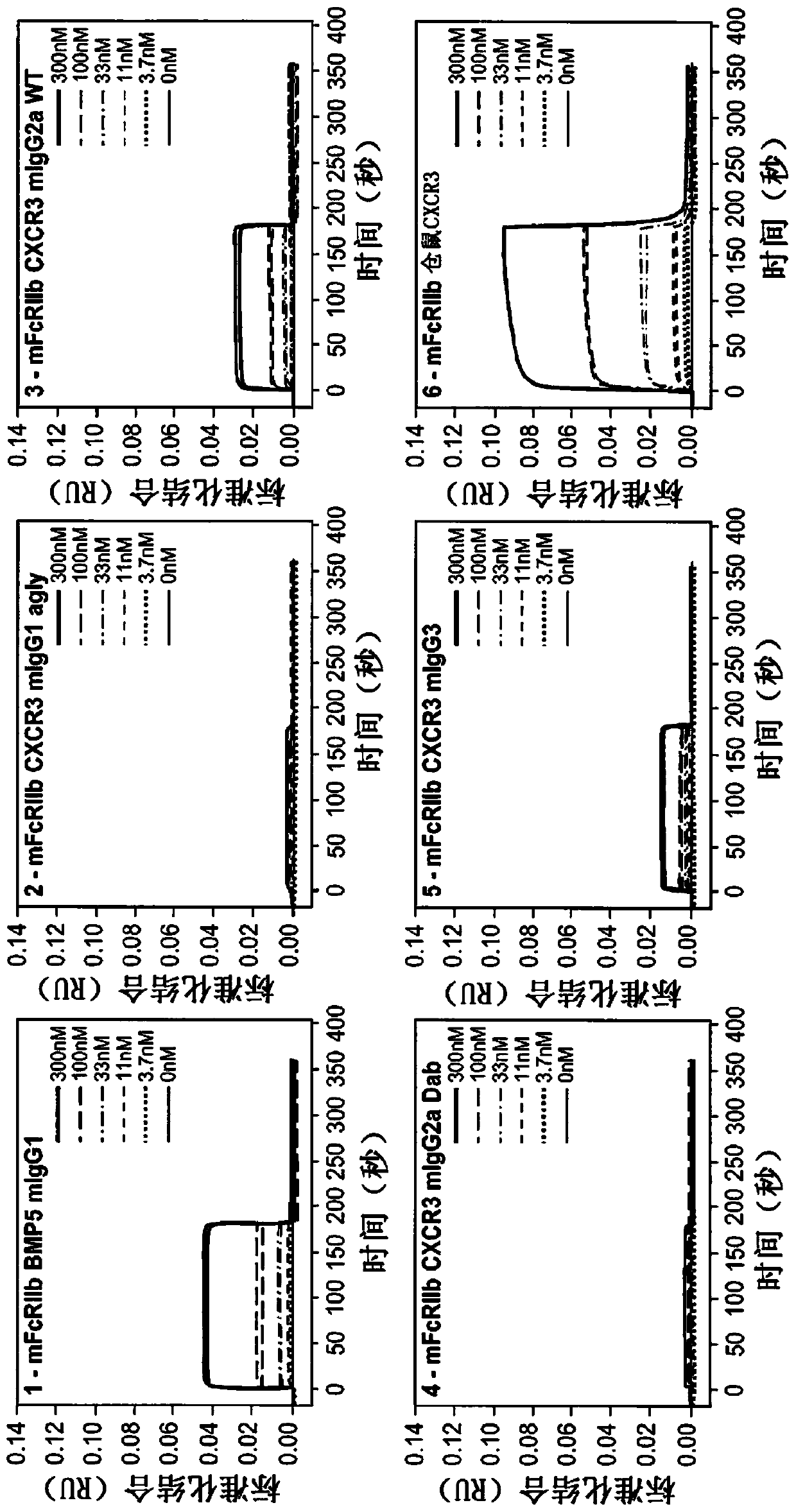
[0325] The following Fc variants of hamster CXCR3-173 mAb were prepared to test the effector function of the antibody: aglycosylated N297G variant of mouse IgG1 (CXCR3 mIgG1 agly); wild-type mouse IgG2a chimera (CXCR3 mIgG2a WT); Modified to eliminate capacity-depleting mouse IgG2a chimera (CXCR3 mIgG2a Dab); and wild-type mouse IgG3 (CXCR3mIgG3).
[0326] C57BL / 6 mice (Jackson Laboratories, n=5 per group) aged 8 to 10 weeks were given a single 5 mg / kg intravenous dose of antibody and blood was harvested 24 hours later for flow cytometry using the following markers Cytometry analysis: CD4 and CD8 T cells were analyzed by TCRab, CD4 and CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com